Figure 10.
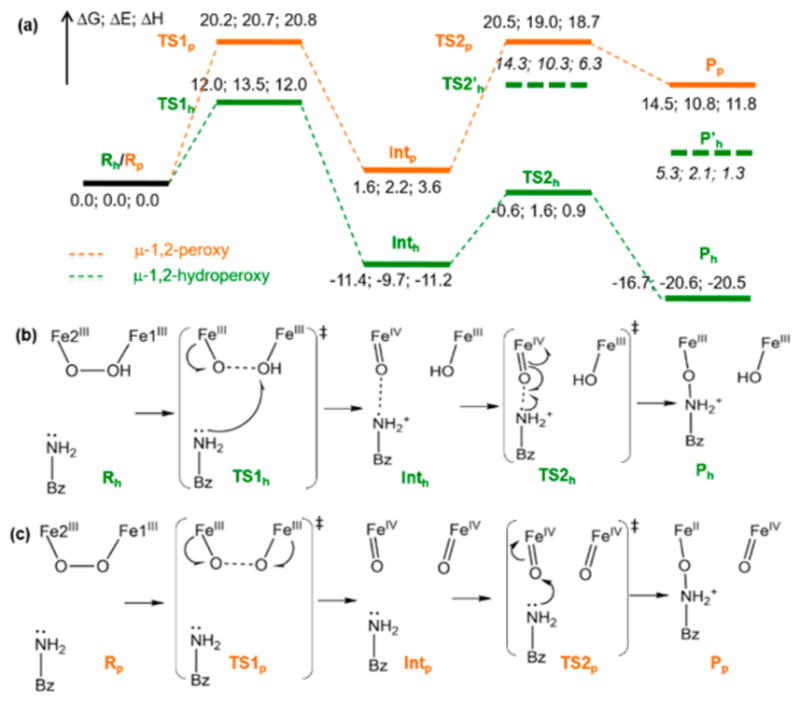
Reaction coordinate for N-oxygenation in AurF. (a) Energy profile (ΔG; ΔE; ΔH) of the AurF μ-1,2-hydroperoxo-bridged P′ intermediate (green) compared to that of a hypothetical μ-1,2-peroxo-bridged P intermediate (orange) for the N-oxygenation of 4-aminobenzoic acid. The dashed energy levels (TS2′h and P′h) are for an alternative unfavorable reaction pathway where the amino group of the substrate forms a bond with the hydroxide on Fe1. (b) Reaction scheme for the P′ intermediate. The NO and OO distances are 3.25 and 1.43 Å in Rh, 3.38 and 1.81 Å in TS1h, 2.66 and 2.82 Å in Inth, 2.00 and 2.86 Å in TS2h, and 1.43 and 2.64 Å in Ph. (c) Reaction scheme for the P intermediate. The NO and OO distances are 3.08 and 1.36 Å in Rp, 3.00 and 1.75 Å in TS1p, 3.01 and 2.60 Å in Intp, 1.80 and 2.79 Å in TS2p, and 1.42 and 2.79 Å in Pp.
