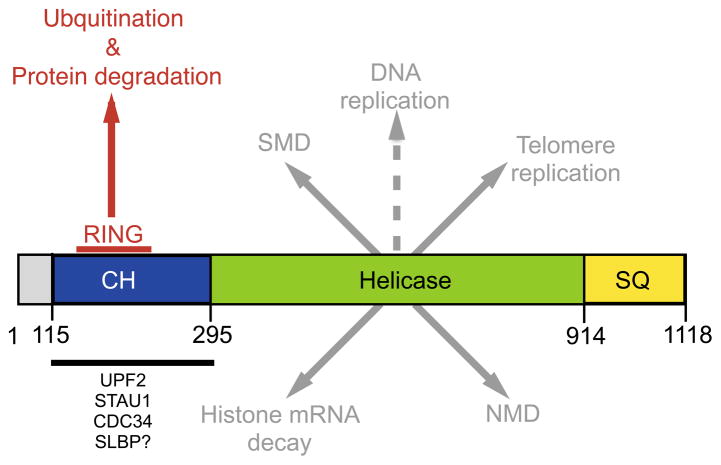
Figure 1.
Domain structure of UPF1 with its various molecular functions highlighted. Domain structure adapted from Dehghani-Tafti et al. [37]. The CH domain is involved in binding several factors (highlighted below the bold line). It is not currently known whether this domain binds SLBP, but seems likely given the similarities between SMD and histone mRNA decay [9]. The RING domain reported by Feng et al. [19] is contained within the CH domain (highlighted by the red line). Mutations within the RING domain disrupt UPF1’s ubiquitination and protein degradation activity [19]. Mutations that disrupt the RNA helicase activity of UPF1 disrupt UPF1’s function in NMD, [38] SMD [39], telomere replication [18] and histone mRNA decay [36]. It has not been directly tested whether the helicase domain is required for UPF1’s putative function in DNA replication, but seems likely given that it is a nucleic acid-dependent process. The SQ domain contains numerous serine residues that are phosphorylated by SMG1 to drive UPF1 function.