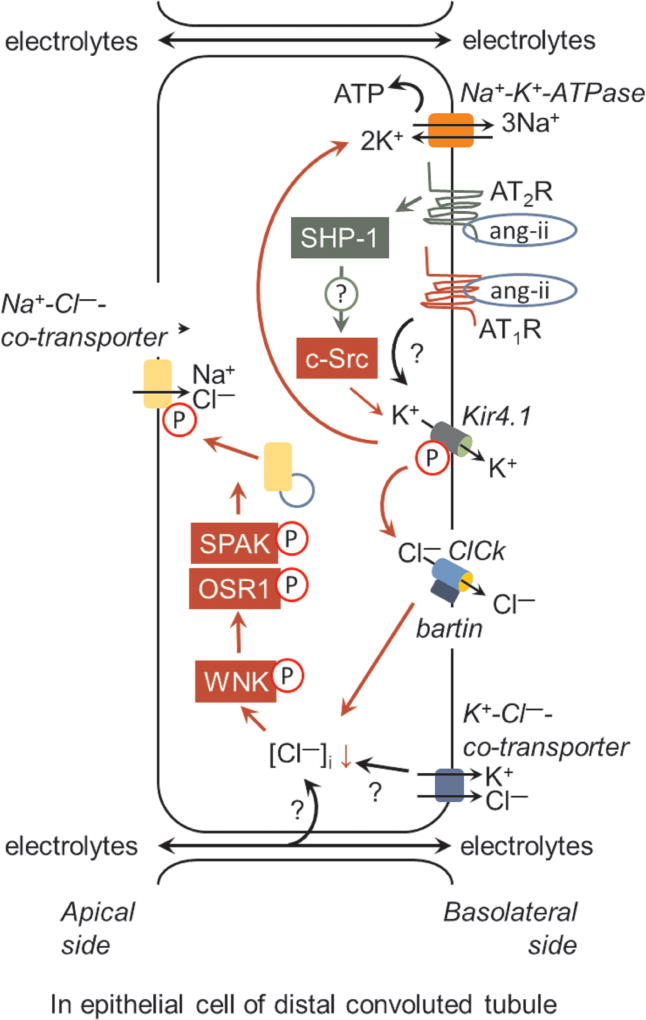
Figure 1.
Role of the angiotensin AT2 receptor (AT2R) in regulation of the apical Na+-Cl− co-transporter (NCC) activity via inhibition of the basolateral 40-pS K+ channel (Kir4.1) activity. The Src family protein kinase, c-Src-, mediated phosphorylation of Kir4.1 is critically required for K+ retrieval. This process facilitates the activity of Na+-K+-ATPase (NKA) for maintaing the cell membrane potential and provides the driving force for Cl− efflux via basolateral Cl− channel (ClCk)-bartin. Cl− efflux results in the decrease of [Cl−]i and hyperpolarization of the basolateral membrane, which is rapidly sensed by with-no-lysine kinase (WNK). This results in the phosphorylation and activation of WNK and phosphorylation of Sterile 20/SPS-1-44-related proline-alanine-rich protein kinase (SPAK)/oxidative stress-related kinase (OSR1). These events are required for vesicular trafficking, phosphorylation, and efficient activity of NCC. However, the following several mechanisms require further elucidation: (a) whether the Src homology region 2 domain-containing phosphatase-1 (SHP-1) is involved in AT2R mediated inhibition of c-Src and decrease in Kir4.1 activity; (b) elucidation of the molecular targets involved in WNK phosphorylation in response to the decrease in [Cl−]i, (c) the role of the K+-Cl−-co-transporter and paracellular transport of anions in the maintenance of the membrane potential and [Cl−]i, and (d) the lack of responsiveness of Kir4.1 to ang-II/AT1R.