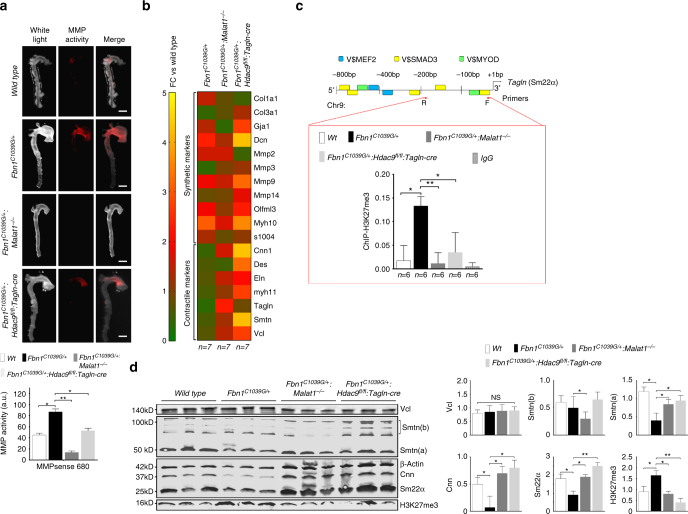
Fig. 7.
Genetic disruption of Malat1 or Hdac9 restores in vivo contractile protein expression. a Malat1 or Hdac9 deficiency decreases in vivo aortic MMP activity in Fbn1C1039G/+ mice. Wild-type, Fbn1C1039G/+, and Fbn1C1039G/+:Malat1−/−, and Fbn1C1039G/+:Hdac9fl/fl:Tagln-cre mice are injected with MMP sense 680 in vivo prior to sacrifice and ex vivo imaging. Bar = 1.5 mm. b Heat map of contractile and synthetic VSMC markers from ascending aortas of six month old Fbn1C1039G/+, Fbn1C1039G/+:Malat1−/−, and Fbn1C1039G/+:Hdac9fl/fl:Tagln-cre mice. Levels shown by fold change (FC) vs. wild type. (n = 7 mice for each genotype). c In vivo chromatin immunoprecipitation (ChIP) assays from mouse aortic tissue (n = 6 mice for each genotype) using H3K27me3 antibodies at the proximal Tagln (Sm22α) promoter. d Malat1 or Hdac9 deficiency rescues decreased expression of multiple VSMC contractile proteins in Fbn1C1039G/+ mice. Western blot of aortas in wild-type, Malat1−/−, Fbn1C1039G/+, Fbn1C1039G/+:Malat1−/−, and Fbn1C1039G/+:Hdac9fl/fl:Tagln-cre mice. Quantification of protein levels of three replicate experiments is shown. (Student’s T-test, NS not significant, *p < 0.05, **p < 0.01) Bar graphs are presented as mean with error bars (±SD). Full-length western blots presented in Supplementary Fig. 9