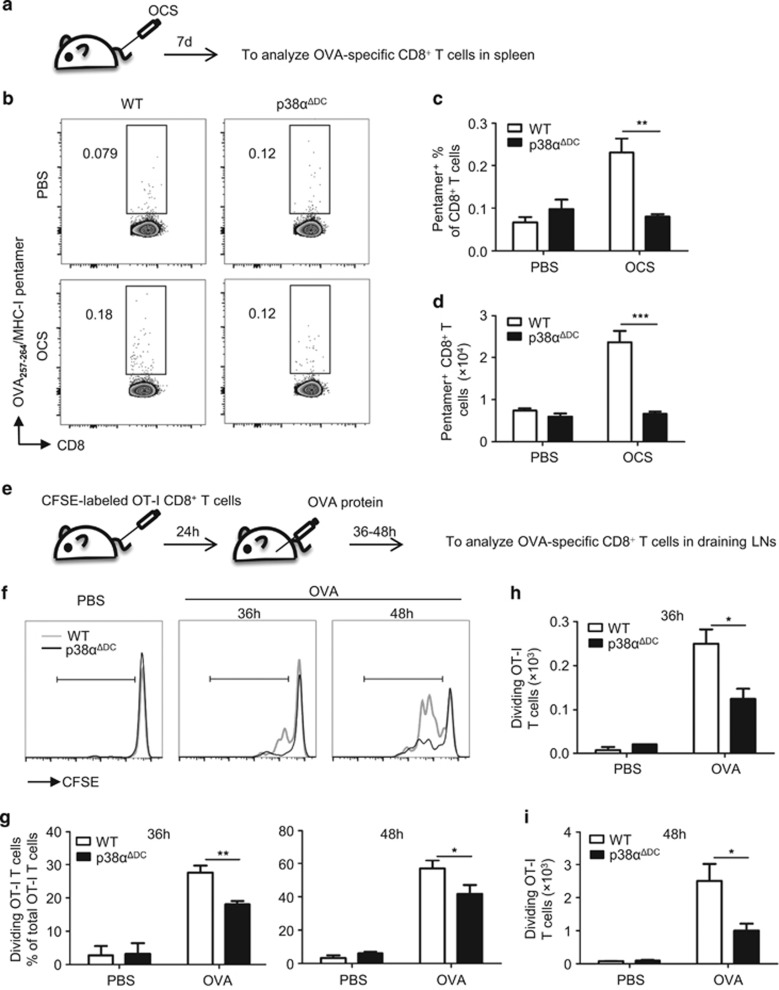
Figure 5.
p38α deficiency impaired cross-priming of T cells in vivo. (a) Splenocytes were irradiated by a 30 Gy X-ray, coated with OVA (1 mg/ml) at 37 °C for 45 min, and then free Ag was washed off. Then, the OVA-coated splenocytes (1 × 107 cells/mouse) were injected into the mice intravenously (i.v.). The proliferation of OVA-specific OT-I CD8+ T cells in the spleens of the injected mice was measured 7 days later using the APC-labeled MHC-I/SIINFEKL pentamer. The percentages (b, c) and numbers (d) of OVA-specific CD8+ T cells in the WT (n=5) and p38αΔDC mice (n=4) are presented. (e) CFSE-labeled OT-I CD8+ T cells (7 × 105cells/mouse) were transferred to the WT and p38αΔDC mice (i.v.). After 24 h, OVA protein (100 μg/mouse) was subcutaneously injected in the recipients at sites near the inguinal lymph nodes (OVA protein, 50 μg/site). A PBS injection was used as a negative control. After 36–48 h, two inguinal lymph nodes were collected from the recipients and analyzed for OVA-specific T-cell proliferation. The percentages (f, g) and numbers (h, i) of OVA-specific CD8+ T cells in the WT and p38αΔDC mice are presented (WT n=5 and p38αΔDC n=5 for the OVA protein-injected group; WT n=2 and p38αΔDC n=2 for the PBS-injected group). The results are presented as the mean±s.e.m. *P<0.05; **P<0.01; ***P<0.001.