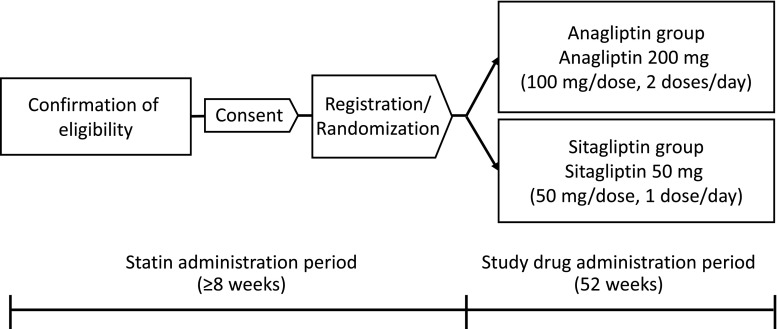
Fig. 1.
Study design. After the statin administration period and informed consent is obtained, eligible patients are randomly allocated to the anagliptin group who receive twice a day anagliptin at 200 mg per day and to the sitagliptin group who receive once a day sitagliptin at 50 mg per day for 52 weeks, the study drug treatment period