Author response image 1. N-terminal Shh processing activates Shh.
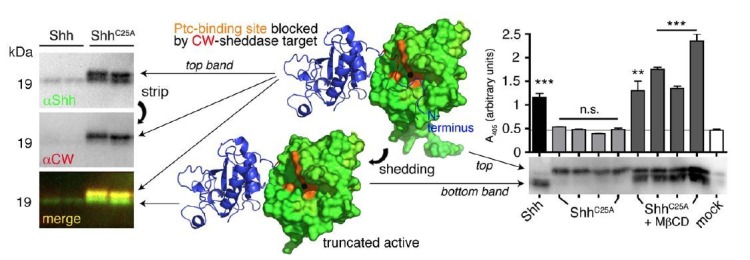
Center: Intermolecular interactions observed in the human Shh crystal structure (pdb:3M1N) (17). Analysis of crystal symmetry mates revealed that N-terminal peptides (amino-terminal amino acids 25-45, blue) wrap around the symmetry-related molecule (green) and interact with its zinc-coordination site, which is the Ptc receptor binding site. Zinc is shown as a black sphere, the yellow surface marks the Ptc-receptor binding site, and the putative sheddase target site (called CW motif) is marked in red. Proteolytic processing during release truncates the protein (bottom) and increases its electrophoretic mobility (left). To better demonstrate N-terminal Shh processing during release, we inverted and colored (gray) the scale blots obtained from α-Shh antibody (binds processed and unprocessed Shh) and α-CW antibody (binds the putative cleavage site) incubation. Yellow signals in merged blots thus denote N-unprocessed proteins bound by both antibodies, and green signals confirm the removal of N- terminal peptides (arrow). Complete processing of N-palmitoylated N-termini (Shh) and incomplete processing of non-palmitoylated termini (ShhC25A) is confirmed by differential α-CW-antibody binding. Right: Protein processing converts otherwise inactive ShhC25A into the active morphogen, as indicated by induced Hh-dependent C3H10T1/2 reporter cell differentiation into osteoblasts. *** denotes statistical significance (p<0.0001), **denotes statistical significance (p<0.0047). n.s.: not significant (<0.05). Taken from (11).
