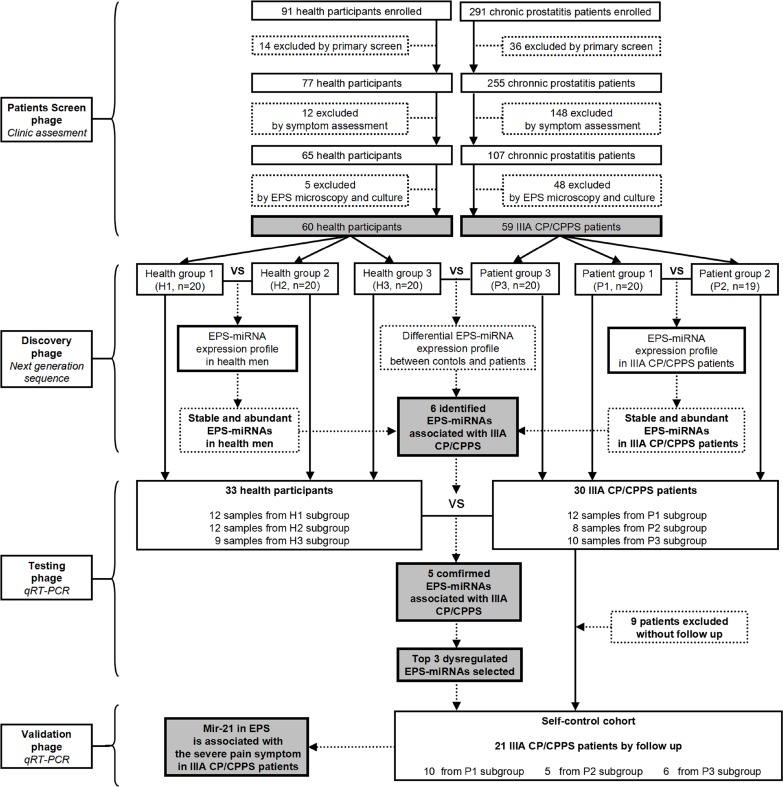
Figure 1. Flow diagram of participant screen and EPS-miRNA identification.
The procedure for identifying EPS-miRNAs consisted of four phages, including patient screen phage, discovery phage, testing phage, and validation phage. In screen phage, 60 healthy men and 59 IIIACP/CPPS patients with significant prostatitis-like pain (NIH-CPSI pain score ≥ 10) were finally included primary screen, symptom assessment, and EPS examination. In discovery phage, high-throughput sequencing was employed to identify characteristic expression-profile of EPS-miRNAs for healthy men and IIIA CP/CPPS patients. In testing phage, elevated levels of identified EPS-miRNAs were further confirmed in the individual EPS samples from 33 patients by comparing to 30 healthy men with Taqman-based qRT-PCR. In validation phage, the change levels of top dysregulated EPS-miRNAs were measured traceably in 21 follow-up patients, and their classify-accuracy on IIIA CP/CPPS were subsequently analyzed by ROC curve.