Abstract
Intestinal neoplasms are common in zebrafish (Danio rerio) research facilities. These tumors are most often seen in older fish and are classified as small cell carcinomas or adenocarcinomas. Affected fish populations always contain subpopulations with preneoplastic lesions, characterized by epithelial hyperplasia or inflammation. Previous observations indicated that these tumors are unlikely caused by diet, water quality, or genetic background, suggesting an infectious etiology. We performed five transmission experiments by exposure of naïve fish to affected donor fish by cohabitation or exposure to tank effluent water. Intestinal lesions were observed in recipient fish in all exposure groups, including transmissions from previous recipient fish, and moribund fish exhibited a higher prevalence of neoplasms. We found a single 16S rRNA sequence, most similar to Mycoplasma penetrans, to be highly enriched in the donors and exposed recipients compared to unexposed control fish. We further tracked the presence of the Myoplasma sp. using a targeted PCR test on individual dissected intestines or feces or tank feces. Original donor and exposed fish populations were positive for Mycoplasma, while corresponding unexposed control fish were negative. This study indicates an infectious etiology for these transmissible tumors of zebrafish and suggests a possible candidate agent of a Mycoplasma species.
Keywords: Zebrafish, Transmission, Intestinal, Neoplasia, Mycoplasma
Introduction
Spontaneously and naturally occurring diseases in laboratory animals provide unique opportunities to investigate mechanisms of disease etiology in a highly controlled model system. For over 15 years, spontaneous intestinal neoplasia has been observed in zebrafish (Danio rerio) submitted to the Zebrafish International Resource Center (ZIRC) diagnostic service. In a retrospective study of the ZIRC data base, we documented such tumors in 2% of about 10,000 fish from 18 zebrafish laboratories submitted between 2000 and 2012 (Paquette et al., 2013), and we continue to see these tumors in diagnostic cases from various zebrafish facilities. The tumors are most often seen in fish older than 1 year, and fish from the same facilities often exhibit preneoplastic changes in the intestine, including hyperplasia, dysplasia, as well as chronic enteritis. The neoplasms appear to be of epithelial origin based on morphology and immunohistochemistry (Paquette et al., 2015), and are consistent with either small cell carcinomas or more rarely adenocarcinomas. The etiology of these common zebrafish tumors is unknown, but several potential mechanisms are unlikely. The high tank to tank variability in disease prevalence across fish held on shared recirculating water systems (where water is circulated through multiple tanks repeatedly) suggests that a water-borne chemical agent is unlikely to be responsible, as such an agent would be expected to quickly disperse across all tanks in the system. When the diet from a laboratory with a high incidence of the disease was fed to zebrafish reared at a different laboratory where no tumors had ever been reported, no pathology was observed in the test fish, and the tumors have been observed in multiple different zebrafish genetic backgrounds (Paquette et al., 2013). Hence, water-born or dietary carcinogens or zebrafish genetics are all unlikely causes.
These combined findings suggest the possibility of an infectious etiology. Numerous neoplastic diseases of fishes have been shown to be caused by transmissible agents and are frequently associated with viruses (Anders & Yoshimizu, 1994; Coffee, Casey, & Bowser, 2013; Getchell, Casey, & Bowser, 1998; Schmale, 1995; Schmale, Gibbs, & Campbell, 2002). The origins of these neoplasms are usually skin epithelium, lymphoid or soft tissue (e.g., sarcomas), but to date no gastrointestinal cancers in fishes have been linked to viruses (Bowser & Casey, 1993; Getchell et al., 1998). In general, parasites that cause chronic inflammation have also been linked to cancer in fish (Samaras, Rafailidis, Mourtzoukou, Peppas, & Falagas, 2010), with several parasites implicated in several cancers, including gastrointestinal neoplasia (Dvir, Clift, & Williams, 2010; Peterson & Weidner, 2011). For example, the nematode Pseudocapillaria tomentosa, a relatively common zebrafish parasite, causes profound chronic inflammation of the intestine (Kent, Harper, & Wolf, 2012; Kent, Bishop-Stewart, Matthews, & Spitsbergen, 2002), and zebrafish exposed to both DMBA and P. tomentosa demonstrate a higher prevalence of intestinal tumors than uninfected fish exposed to DMBA (Spitsbergen et al., 2000). While this nematode was implicated in the original diagnosis of several affected fish, it was not prevalent amongst the affected laboratory zebrafish in our retrospective study (Paquette et al., 2013).
Within mammals, gastrointestinal neoplasms are often linked to bacterial agents (Sears & Garrett, 2014), particularly Helicobacter pylori, which is the most common cause of gastric cancer (Peek, 2016). Other bacteria that have been suggested to contribute to GI neoplasms include Fusobacterium nucleatum, enterotoxigenic Bacteroides fragilis, and colibactin-producing Escherichia coli (Brennan & Garrett, 2016). While bacteria have not previously been associated with gastrointestinal neoplasms in fishes specifically, we previously showed that medaka, Oryzias latipes, exposed to benzo-a-pyrene have an increased incidence of liver tumors when co-infected with Mycobacterium marinum (Broussard et al., 2009). Other evidence points to pathologic shifts in the resident gut bacteria, referred to as dysbiosis, as an instigator or driver of gastrointestinal cancers. Multiple studies have reported altered microbiota in colon cancer patients versus healthy controls and even in tumor versus adjacent, healthy tissue biopsies (Louis, Hold, & Flint, 2014). Experimentally, mice deficient for the NOD-like receptor family pyrin domain containing 6 (NLRP6) gene develop inflammation-associated colorectal cancer that is transmissible to co-house wild type mice (Hu et al., 2013). We demonstrated in gnotobiotic zebrafish that excessive intestinal epithelial cell proliferation in zebrafish larvae with an oncogenic mutation in axon1 was reduced in the absence of microbiota and enhanced by the presence of particular bacteria (Cheesman, Neal, Mittge, Seredick, & Guillemin, 2011). Therefore, a bacterium or perhaps a consortium of bacterial species are reasonable candidates as the cause for the zebrafish intestinal neoplasia. Although experimental evidence has not linked specific bacteria to carcinogenesis in zebrafish to date, the chronic inflammation elicited by certain pathogenic strains of bacteria, and even the natural microbiota, of zebrafish could potentially serve as promoters of intestinal carcinogenesis.
The possibility of an infectious etiology combined with the evidence against diet, water born carcinogenic chemicals, or genetics as likely causes suggests these tumors could be spread among zebrafish populations through exposure to a shared environment. We performed a series of experiments with the primary goal of testing whether this intestinal neoplasm could be transferred from afflicted to healthy zebrafish populations through co-habitation. In addition, we also assessed the composition and distribution of intestinal bacteria in zebrafish in the study to see if changes in the intestinal microbiota were linked to disease transmission. This combined approach allows us to test the transmissibility of the disease and to generate hypotheses and tools to further investigate the etiology of the disease. We also observed liver lesions in some populations exposed to donor fish, and these data are presented in the Supplement.
Methods
Ethics statement
All zebrafish experiments were done in accordance with protocols approved by the Oregon State University and the University of Oregon Institutional Animal Care and Use Committees and conducted following standard protocols as described (Westerfield, 2007).
Experimental Transmission
Approximately 200 one-year-old zebrafish from a laboratory with a high prevalence of the intestinal tumors, previously designated as “primary facility” (Paquette et al., 2013), were transferred to our laboratory in Nash Hall, Oregon State University and used to establish a series of transmission experiments to assess the transmissibility of the intestinal tumors across zebrafish populations. These donor zebrafish, and all other experimental animals, were maintained on a single pass flow-through system in which the incoming water is city source, dechlorinated with activated carbon, and heated to 28 C. A total of five transmission experiments were performed in which healthy recipient zebrafish were either cohoused in a shared tank with primary (fish from the primary facility) or secondary (originally healthy recipients exposed to primary donors) donor fish (Exposures A, B, and E) or exposed to unfiltered effluent from tanks housing primary or secondary donor fish (Exposures C and D) for between four and twelve months each (Figure 1a). The primary donor zebrafish represented a mixture of three populations of wild type AB background fish and ranged in age between 315 to 441 days post fertilization (dpf), while healthy recipient fish alternated between similarly aged Casper zebrafish lacking pigmentation (Exposures A, B, and E) and wild type outbred 5D zebrafish (Exposures C and D) to allow for simple visual differentiation of donor and recipient individuals. In addition, for each transmission experiment, zebrafish from the same population as the recipients were maintained under similar conditions but not exposed to donor fish to act as controls. Details about each individual transmission experiment are as follows:
Figure 1.
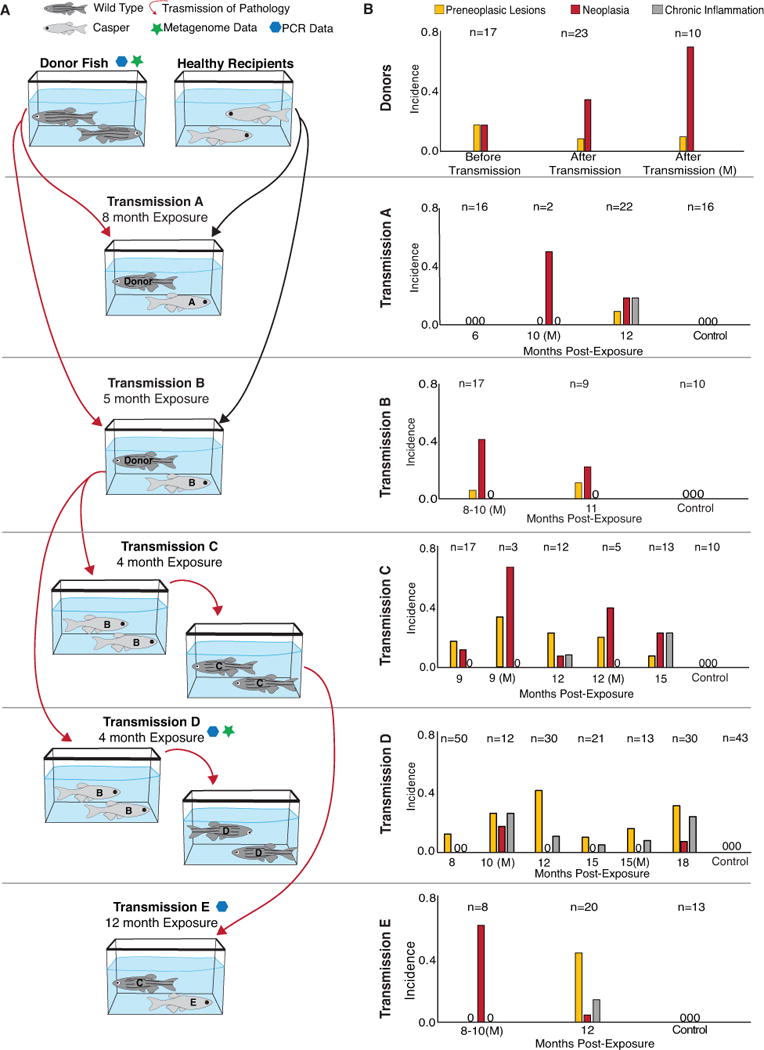
A. Transmission paradigm. Donor fish with high levels of intestinal tumors were co-housed with healthy casper fish (Exposures A and B). After exposure to donors, transmission of disease was propagated by exposing healthy 5D fish to effluent from tanks of Exposure B fish (Exposures C and D). After Exposure C was exposed to Exposure B, Exposure C fish were co-housed with healthy Casper fish (Exposure E). Red arrows indicate transmission of pathology from one group to another. B. Incidence of intestinal lesions in donor and recipient zebrafish. Preneoplastic designates hyperplasia or dysplasia of epithelium. All fish with neoplasia had preneoplastic lesions in other regions of the intestine, but these fish were not included in the “preneoplastic” data. M = moribund fish. PCR + or – indicates samples positive or negative Mycoplasma PCR test.
Exposure A: A total of 175 donor fish were place in a 100 L circular tank. Then three months later 58 recipient fish (three week old Casper line obtained from Children’s Hospital), were exposed to effluent from the donor fish tank for two weeks due to their small size and then transferred to the tank with the donor fish and cohabitated with the donor fish for an additional seven and a half months.
After a total of eight months exposure, the recipient fish were removed and maintained for an additional four months in a separate 16 L tank. Sixteen control fish from the same population were maintained on the same water system and fed the same diet, but not co-habitated with the donor fish. Six of these fish were examined at six months post-exposure, and the remaining ten were examined at twelve months post-exposure (Figure 1b).
Exposure B: Fifty adult Casper zebrafish from the Sinnhuber Aquatic Research Laboratory (SARL) were exposed to seventy of the donor fish in a 16 L tank for five months, and then the donor fish were removed. Ten control fish from the same population were maintained on the same water system and fed the same diet, but not co-habitated with the donor fish. These fish were examined at eleven months post-exposure.
Exposure C: A total of 50 5D fish were exposed to the effluent of a tank holding Exposure B (as donor fish) for 3 months. The effluent flowed at approximately 1L per 5 min. The recipient tank water was supplemented with fresh, unexposed water at a rate of 1L per 2.5 min to maintain water quality. Recipient fish (Exposure C) were examined at 9, 12 and 15 months post-exposure, and moribund fish were examined at 6 and 9 months post-exposure (Figure 1b). Ten control fish (unexposed) from the same population were examined at 12 months post-exposure.
Exposure D: As with Exposure C, 5D fish were exposed to effluent from Exposure B for 4 months. In this exposure trial, the 300 recipient fish were divided into three 16 L tanks, receiving the effluent from Exposure B in the same manner as Exposure C. Recipient fish were examined at 8, 10, 12 and 15 months post-exposure. In addition, several emaciated fish were collected and examined at 10, 15, and 18 months post-exposure (Figure1b). Control fish were examined as follows at 10 (n=10), 12 (n=8), 15 (n =10), and 18 (n=15) months post-exposure.
Exposure E: This group represented fish exposed to Exposure C. Here a total of 37 Casper fish (starting age of 6 months) were cohabitated with Exposure C (as donor fish) for 12 months. Sixteen control Casper fish from the same population were also examined at 13 months post-exposure.
Histology and analysis of disease prevalence
Following each transmission experiment, recipient zebrafish were removed from the exposure treatment and maintained in separate tanks. The intestines of individual fish were then sampled for histopathology, while some collections involved histological examination of the entire fish. Fish were euthanized by icing (Matthews & Varga, 2012), the abdomen was then opened with and longitudinal cut, and whole fish were preserved in Dietrichs solution, Fish were processed for histology with sagittal cuts prepared from whole fish, and slides were then stained with hematoxylin and eosin.
The pathological changes in the intestine of each fish was designated as inflamed, preneoplasic (including fish with hyperplasia or dysplasia of the intestinal epithelium), neoplastic (either small cell tumors or adenocarcinomas) or normal as described by Paquette et al. (2013). All fish with neoplasms exhibited preneoplastic tissue regions in other regions of the intestine. A Cochran-Mantel-Haenszel chi-squared test of independence (CMH test) was used to determine whether exposed fish were statistically enriched for the development of neoplasia relative to control fish while controlling for the exposure group covariate.
At multiple time points post-exposure, a subset of fish from the donor population as well as Exposures D and E were selected for bacterial DNA analysis in addition to histopathology. For these fish, the anterior half of each intestine was processed for histology to assess disease state while the posterior half was retained for DNA analysis (see below). Here fish were dissected to expose the coelomic cavity and the anterior half of the intestine was preserved in Dietrich’s fixative and processed for histology with multiple slides prepared for each piece of intestine. To verify that histopathology of the anterior portion was sufficient to accurately diagnose fish, we reexamined the slides and the raw data reports from our previous retrospective study (Paquette et al., 2013). Of the 194 fish in this previous study with either tumor or preneoplastic lesions, none shown lesions confined to the posterior intestine.
Intestinal Microbiome Profiling
In order to assess a possible relationship between disease occurrence and bacterial communities, we sampled and characterized the intestinal bacterial microbiota of zebrafish from both the beginning of the transmission experiment, the donors, and from the end, Exposure D, using high-throughput sequencing of the 16S rRNA gene. Donor fish were sampled immediately following Exposure B, while Exposure D fish were sampled 15 months post-exposure. The posterior intestines of 42 donor fish, 30 Exposure D fish, and 19 control fish (5 controls for donors and 14 for Exposure D) were removed aseptically, placed in a 2mL screw-cap tube and stored at -80°C prior to subsequent processing, while anterior portions were preserved for histology (described above). Each transmission trial lasted over a year, and hence the overall study spanned several years. Due to changes in the technology and methodology available over the course of the entire transmission experiment, donor and Exposure D samples were processed using different methods from one another. DNA was extracted from donor samples using a combination of bead beating and the Qiagen DNeasy Blood and Tissue Kit as described by Stephens et al. (Stephens et al., 2016), while DNA from Exposure D samples was extracted using MoBio PowerMag RNA/DNA Isolation Kit. The V4 region of the 16S rRNA gene was amplified using the 515F and 806R primers (Caporaso et al., 2012). Amplicons were then sequenced using the Illumina NextSeq platform for donor samples and the Illumina HiSeq 2500 platform for Exposure D samples generating 150 base pair paired-end sequences from both. Sequences were then assembled using FLASH (Magoc & Salzberg, 2011) and quality filtered using the FASTX Toolkit (Hannon Lab, 2010). Host sequences were filtered from the dataset by aligning reads to the zebrafish genome using Bowtie (Langmead & Salzberg, 2012). Sequences from both runs were then combined to generate operational taxonomic units (OTUs) de novo at 97% sequence similarity using USEARCH (Edgar, 2010). The taxonomy of these OTUs was then assigned using the RDP classifier (Wang, Garrity, Tiedje, & Cole, 2007). Read assembly, quality filtering, and OTU clustering were done on the University of Oregon ACISS cluster. The resulting OTU table was rarefied to 40,000 sequences per sample, and downstream community analyses were then performed in R (R Core Team, 2016) using the vegan (Oksanen et al., 2016) and DESeq2 (Love, Huber, & Anders, 2014) packages.
Mycoplasma PCR
Following results suggesting that a Mycoplasma OTU was predominant in fish that had lesions (described in the Results section below), we adapted a genus specific PCR test (van Kuppeveld et al., 1994) to determine the presence of Mycoplasma in fecal samples from tanks during the experiment and to correlate the presence with 16S rRNA gene profiling data. Microbial DNA was isolated from original donor fish intestines and fecal samples from tanks of exposed and unexposed control tanks were analyzed. Fecal samples were collected from tanks of fish, centrifuged and DNA was extracted using PowerLyzer PowerSoil DNA Isolation Kit (Mo Bio) following manufacturer protocol. 2 μL of DNA isolated from fecal samples and intestinal samples were used as a template in a 25 μL reaction containing 0.25 μL Phusion HF polymerase (Thermo Scientific), 5 μL 5× Phusion GC Buffer (Thermo Scientific), 0.4mM dATP, dTTP, dCTP and dGTP, and 400nM of each primer, in 14.74 μL nuclease-free water with the following cycle conditions: 98° C 30 seconds, 35 cycles of 98°C for 10 seconds, 55.3°C for 15 seconds, and 72°C for 30 seconds. 70 °C for 5 minutes, reaction held at 4°C. 5 μL PCR product was mixed with 1 μL 6× DNA loading dye. Run on 1% agarose gel stained with ethidium bromide. Gel visualized with UV light. Presence or absence of band at 280 bp indicated presence or absence of Mycoplasma species in the sample. Mycoplasma group-specific primer set, amplifies 280-bp fragment. Forward Primer GPO-3 (5′-GGGAGCAAACAGGATRAGATACC CT-3′) and the reverse primer MGSO (5′-TGCACCATCTG TCACTCTGTTAACCTC-3′) (van Kuppeveld et al., 1994). Amplicons were extracted from agarose gel, purified using Zymo Gel DNA Recovery kit, and sequenced to verify that sequences from Mycoplasma were amplified.
To validate the sensitivity of the PCR assay, the 280 bp fragment amplified by PCR from Exposure D fecal DNA was gel purified and subcloned into an ampicillin-resistant E.coli plasmid with synthetic multiple cloning site pGEN-MCS (Lane, Alteri, Smith, & Mobley, 2007) via smaI site and transformed into DH5α cells. The plasmid was amplified by growing overnight in a 5 mL culture in LB containing ampicillin and purified using Zymo Plasmid Miniprep Kit. The number of plasmid copies present in sample was then calculated. PCR was performed as described earlier testing a series of plasmid dilutions. The mean copies of plasmid present to generate a positive result by PCR is 517.23 (geometric mean, ranging from 93.7 to 8700 copies).
Results
Approximately two hundred zebrafish from a laboratory with a high prevalence of intestinal tumors were used to establish a series of transmission experiments to assess the transmissibility of the intestinal tumors across zebrafish populations (Figure 1a). The main goals of the series of experiments was to (1) assess the transmissibility of the intestinal tumors, (2) assess whether the disease could be transmitted over multiple “generations” of exposure, and (3) determine whether exposure to effluent tank water was sufficient to transmit the disease or if direct contact between populations was required.
Preneoplastic lesions and neoplasms were observed in all six groups of recipient fish as well as the donor fish (Figure 1b; n=350). Chronic inflammation was also observed in most of the exposed fish populations. The lesions were consistent with those seen in our retrospective study of affected zebrafish (Paquette et al., 2013), and are described in detail in Figures 2 and 3. These intestinal phenotypes were not observed in any of the control fish for any transmission experiment (n=102). Indeed, a test of independence (Cochran-Mantel-Haenszel, or CMH, chi-squared test) found that there was a significant increase in the frequency of fish that developed neoplasia in the exposed groups relative to the control groups (p=0.00049). Likewise, all exposure groups had a greater frequency of preneoplastic lesions, and a CMH test similarly identified a significant enrichment in total intestinal lesions among exposed fish relative to controls (p=7.9e–10).
Figure 2.
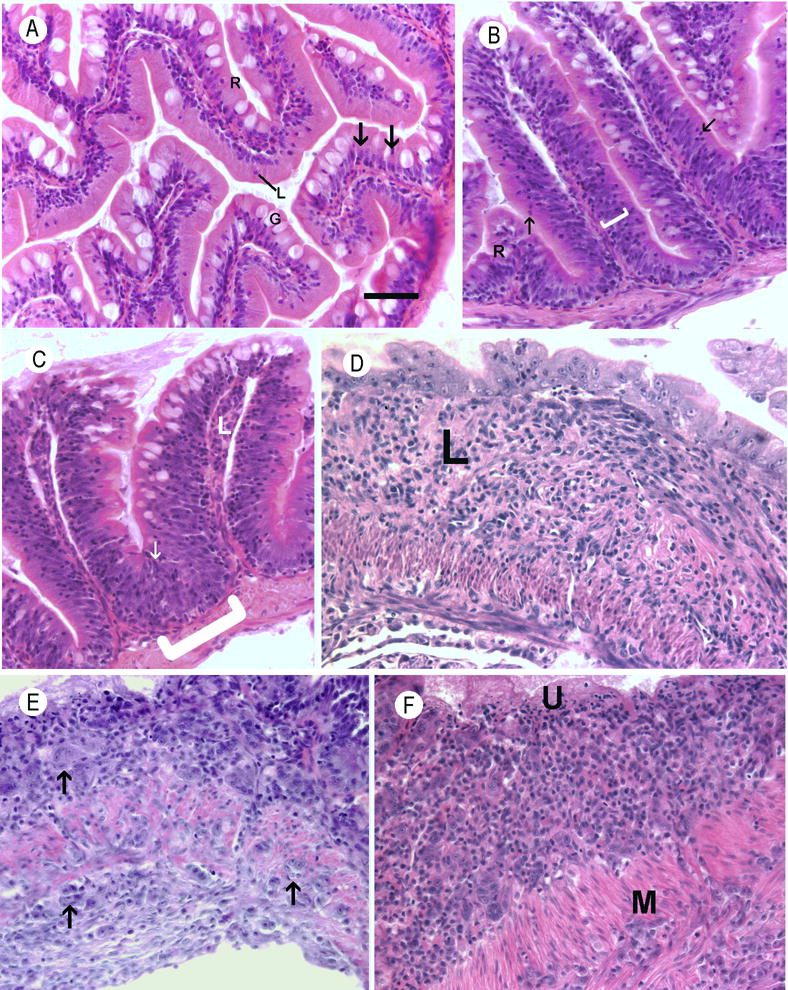
Histological sections of zebrafish intestines. Hematoxylin and eosin. Bar = 25 μm. A. Normal intestine. Nuclei are basal, lymphocytes (L) are uncommon within the epithelium, and goblet cells (G) were frequently observed. B. Moderate epithelial hyperplasia in an exposed fish from group A. The layer of basal located nuclei within the mucosal epithelium is diffusely thickened (bracket) and there are numerous intraepithelial leukocytes composed primarily of lymphocytes (arrows) C. Severe epithelial hyperplasia from an exposed fish from group A. The layer of basilar nuclei is severely thickened and extends to near the brush border in some places (arrow). At the bases of intestinal folds, the mucosal epithelium is markedly thickened and bulges into the muscularis (bracket). D. Chronic severe lymphoplasmacytic and fibrosing enteritis in an exposed fish from group D. The lamina propria (L) is severely thickened by loosely organized fibrous connective tissue containing an inflammatory infiltrate Intestinal folds have been blunted or effaced, and the luminal aspect is lined by a layer of severely dysplastic epithelial cells. E. Small cell intestinal carcinoma from in an exposed fish from group B. Expanding the lamina propria is a poorly-demarcated, unencapsulated neoplasm composed of nests and clusters of polygonal cells (arrow) embedded in a scant fibrovascular stroma. Neoplastic cells have variably distinct cell borders with large amounts of infrequently granular, lightly basophilic cytoplasm. The neoplastic cells invade the tunica muscularis (M) and are present on the serosal surface of the intestine(S). F. Small cell carcinoma in an exposed fish from group B. The lamina propria contains large numbers of neoplastic epithelial cells similar to those described in image E. The overlying epithelium is ulcerated (U). Neoplastic cells invade through the tunica muscularis (M).
Figure 3.
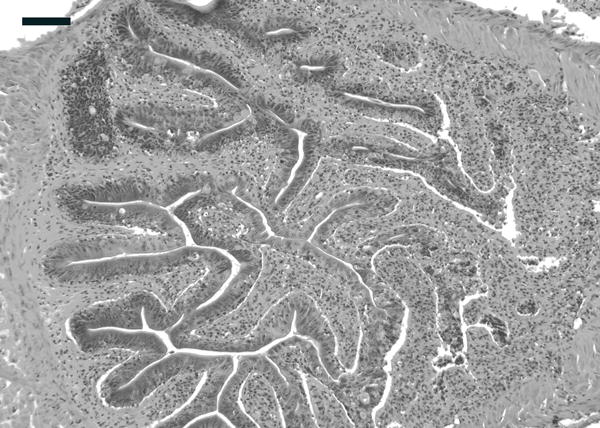
Prominent, chronic enteritis in anterior region of intestine, with diffuse inflammatory infiltrate in the lamia propria. Intestine also exhibits moderate epithelial hyperplasia. H&E. Bar = 50 μm.
Multiple moribund fish were observed over the course of the transmission experiments and examined at the time of clinical presentation (Figure 1b). These moribund fish in both the donor group and the recipient groups had a higher prevalence of lesions, and thus we analyzed the results further to determine whether the morbidity was associated with the disease. Grouping all donor and recipient fish together, 32% (34/106) of the moribund fish had intestinal lesions (either preneoplastic, enteritis or neoplasia) and 57% (25/44) exhibited neoplasia. In contrast, only 17% (36/218) or 16% (45/280) apparently healthy fish had intestinal lesions or evidence of neoplasia, respectively. We then used a Fisher’s exact test of independence to determine if the frequency of moribund or dead fish that exhibit neoplasia is significantly greater than the corresponding frequency among apparently healthy fish. Doing so, we found very significant associations between morbidity and intestinal lesions in general (p = 0.0014) and even more so with neoplasia (p=2.95e-08). Moribund fish not exhibiting intestinal lesions or neoplasms frequently had systemic mycobacteriosis, including three in Exposure B, one in Exposure C, one in Exposure D, and one in Exposure E.
Intestinal microbiotas of donor and exposed zebrafish are enriched for Mycoplasma
Given the evidence that this disease is transmissible across zebrafish populations, we sought to investigate whether there was a relationship between the intestinal microbiota and either disease incidence or exposure, which would implicate a bacterial agent being involved in the etiology of this disease. We obtained 16S rRNA gene profiles of gut microbiota from affected and normal zebrafish to identify candidate bacterial taxa associated with the disease, using similar approaches to those we have employed to characterize the intestinal microbiota of healthy zebrafish (Roeselers et al., 2011; Stephens et al., 2016). Of the 42 donor fish sampled for microbiota analysis, 12 were diagnosed with preneoplasia and 4 with neoplasia, while of the 30 Exposure D fish sampled, 8 were preneoplasic and only 2 had developed full neoplasia. This is in contrast to both groups of controls, none of which showed signs of the disease. Across the entire dataset, we observed a small but significant difference in bacterial composition between healthy and diseased (both preneoplasic and neoplastic) samples (PERMANOVA: pseudo-F = 1.492, p < 0.05), as well as a much stronger difference between exposed populations (i.e. donor and Exposure D recipient fish) and their respective controls (PERMANOVA: pseudo-F = 3.24, p < 0.001 for donors compared to controls and pseudo-F = 7.62, p < 0.001 for Exposure D recipients compared to controls). These overall differences in composition were largely driven by a single OTU belonging to the Mycoplasma genus: one of only two OTUs (the other belonging to the Vibrio genus) that was significantly differentially abundant in diseased compared to healthy individuals (Figure 4a; log2-fold change = 3.46, p < 0.001), and which most strongly differentiated exposed samples (i.e. donor and Exposure D fish) from controls (Figure 4b; log2-fold change = 11.37, p << 0.0001). The average abundance of Mycoplasma was similar amongst the exposed fish, regardless of whether they had lesions or were normal (Figure 4a). The relatively low number of fish sampled with neoplasia made it difficult to identify significant correlations between bacterial abundance and disease severity (i.e. preneoplasia vs. neoplasia), such that there was no significant relationship between abundance and disease severity for any OTU in the dataset.
Figure 4.
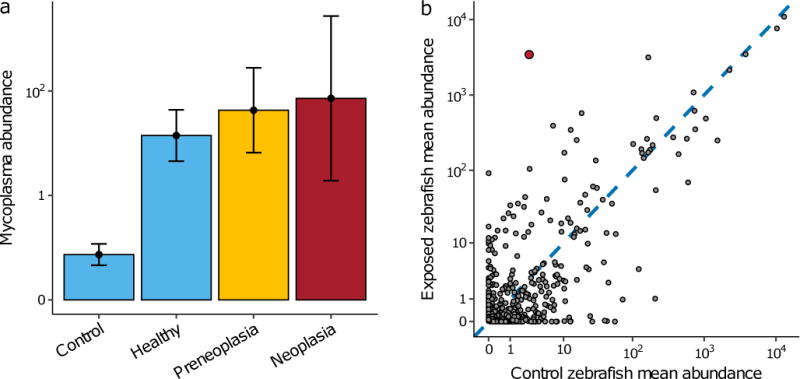
Distribution of bacterial taxa in healthy and diseased fish. A. Average abundance of a Mycoplasma OTU in healthy and diseased fish across the dataset. B. The mean abundance of each bacterial OTU in control zebrafish (x-axis) and exposed (both donor and Group D) zebrafish (y-axis) across the dataset. The red point denotes the OTU belonging to the Mycoplasma genus and most strongly differentiates exposed and control populations.
The observation of a single OTU that strongly differentiated both exposed populations as well as the occurrence of the disease motivated a more focused investigation of this Mycoplasma strain using targeted PCR (see methods). Mycoplasma was present in intestinal samples from the donor group and fecal samples from Exposure D and E by PCR (Figure 1b). Mycoplasma was not detected in the unexposed control groups that were tested (Exposure D and E). Furthermore, in donor samples which indicated a high prevalence of Mycoplasma by 16S rRNA gene profiling, brighter bands were visible on the agarose gel compared to individuals with lower levels of Mycoplasma.
Whereas most of the other dominate phyla and classes of bacteria in the dataset were comprised of multiple lower level taxa (for example, we detected over 70 genera belonging to the class Gammaproteobacteria), this single Mycoplasma OTU was the only member of the class Mollicutes that was detected. Furthermore, despite being relatively dominant numerically, the entire Mycoplasma OTU was comprised of only a single unique sequence, suggesting that it represents a single unique strain, or at least species, of bacteria. This sequence most closely matched a 16S sequence belonging to a Mycoplasma penetrans strain isolated from salmon intestines (Holben et al., 2002).
Discussion
These exposure trails, comprised of five populations of zebrafish, three independent “generations” of transmission, and both cohousing and effluent exposure transmission modes, provide compelling evidence that a common intestinal neoplasm of zebrafish is caused by a transmissible agent. Tumors were observed in recipient fish from all trials when examined starting at nine months post-exposure, following exposure by cohabitation or effluent water. Neither preneoplastic lesions nor the tumors were observed in control fish. Moreover, these lesions have never been documented in hundreds of other zebrafish used in other experiments in our laboratory at Oregon State University where the experiments were conducted, including those of the Casper and 5D lines held in the same OSU laboratory and fed the same diet. The pathologic changes observed in the zebrafish in this transmission study was similar to that observed in a retrospective study of some 10,000 zebrafish examined over a 12 year period from several other facilities and in several fish lines, particularly in fish that were older than one year (Paquette et al., 2013). We have observed morphologically similar intestinal neoplasms in transgenic zebrafish that overexpressed the Helicobacter pylori virulence factor CagA and were homozygous for a loss-of-function allele of p53 (Neal, Peterson, Kent, & Guillemin, 2013), suggesting that these neoplasms may arise from common carcinogenic processes.
The lesions, particularly neoplasms, were associated with increased morbidity. The association of lesions with disease was more profound than observed previously (Paquette et al., 2013). This previous study actually found a higher prevalence of the preneoplastic or neoplasms in apparently healthy, sentinel fish than those submitted as clinical. One explanation for this difference is that the fish examined by Paquette et al. (Paquette et al., 2013) included many diagnostic cases with moribund fish that had succumbed to a variety of infectious diseases that are common in zebrafish, such as mycobacteriosis (Whipps, Lieggi, & Wagner, 2012) or microsporidiosis caused by Pseudoloma neurophilia (Sanders, Watral, & Kent, 2012). A few moribund fish from Exposure B and one healthy fish from Exposure D exhibited granulomas consistent with mycobacteriosis in the coelom or kidney. Pseudoloma neurophilia was observed in on moribund fish from Exposure G. This probably represented a background infection from the original donor fish or the recipient fish from Children’s Hospital in group A, as all of the other the recipient fish were specific-pathogen free (SPF) for this parasite (Kent et al., 2011). Beyond this single case, we did not detect this, or any other pathogenic nematode, in either donor or recipient fish, suggesting they are not the primary cause of these neoplasms.
In our study, donor and recipient fish were comingled in the same tanks or exposed to effluent that was not filtered, and thus we cannot exclude the possibility of actual transfer of neoplastic cells, as seen with in transmissible venereal tumors in dogs and facial tumors in Tasmanian devils (Murchison, 2008) and hemic neoplasms of bivalve mollusks (Metzger, Reinisch, Sherry, & Goff, 2015). However, the high prevalence of concurrent diffuse epithelial hyperplasia in the affected populations without neoplasms suggests that the tumor arise from preneoplastic changes in host cells rather than from direct transfer of foreign neoplastic cells.
The most compelling evidence from our study implicates a strain of Mycoplasma as a candidate etiological agent of the lesions. A single strain of Mycoplasma sp. was enriched in populations exposed to diseased individuals compared to controls, and PCR analyses with the Mycoplasma genus specific test consistently yielded positive results in exposed fish, but not controls. While control versus exposed fish showed distinct differences in Mycoplasma abundance, the abundance was similar within exposed fish regardless of their lesion status. This is not surprising as gut bacteria could be readily shared amongst fish within a tank due to oral-fecal transmission. This is a plausible candidate to pursue as Mycoplasma spp., frequently employ intracellular lifestyles (Rottem, 2003), cause a variety of pulmonary and urogenital infections (McGowin & Anderson-Smits, 2011; Waites & Talkington, 2004), and have been linked to cancers (Rogers, Rogers, & B., 2011), including associations with intestinal cancers (Barykova et al., 2011; Mariotti et al., 2010; Yang et al., 2010) and induction of malignancy in cell cultures (Feng, Tsai, Rodriguez, & Lo, 1999; Namiki et al., 2009). Mycoplasma spp. are frequently dominant in the intestines of adult, but not juvenile or larval, salmon in the wild (Llewellyn et al., 2016), but they have only rarely been associated with disease in fish. A novel species of Mycoplasma, Mycoplasma mobile, was previously isolated from the gills of tench (Tinca tinca) fish with “red disease” and subsequently characterized (Kirchhoff et al., 1987; Kirchhoff & Rosengarten, 1984). This bacterium was later shown to be able to infect host cells and experimental exposure of M. mobile to tench resulted in gill epithelial necrosis (Stadtländer, Lotz, Körting, & Kirchhoff, 1995; Stadtländer & Kirchhoff, 1990).
We have previously detected Mycoplasma 16S rDNA gene sequences in a survey of healthy zebrafish in the University of Oregon facility, especially in elderly fish at 300 dfp (Stephens et al., 2016), but in these cases we observed a diversity of mostly rare sequences, in contrast to the single abundant sequence we detected within the affected donor and recipient fish populations. The apparent clonal nature of the Mycoplasma combined with its frequent dominance compared to other bacterial taxa in the transmission studies supports the hypothesis that it is an etiological agent of the lesions, rather than a background group of bacteria that opportunistically proliferates in zebrafish with intestinal lesions. We have seen the same phenomenon with Mycobacterium marinum outbreaks in hybrid striped bass, where one genotype based on RFLP analysis persisted at a farm for several years (Ostland et al., 2007), and one genotype of Mycobacterium chelonae predominated the infections at a large zebrafish facility (Whipps et al., 2012).
Whereas the agent has yet to be confirmed, we recommend that veterinarians and technicians manage zebrafish with these intestinal lesions (Paquette e al. 201) as a communicable disease. Though far from conclusive, preliminary evidence highlights a strain of Mycoplasma as a potential agent warranting further investigation. We are not, however, excluding the possibility of a different agent, such as oncogenic viruses, as the primarily cause. This should be considered as cancers often require more than one factor to develop. Furthermore, bacterial profiles associated with gastro-intestinal lesions may be the result of the pathological change, rather than the underlying cause (Garrett, 2015; Wroblewski, Peek, & Coburn, 2016). Nonetheless, the association of Mycoplasma with exposed populations of zebrafish opens up additional avenues to study the transmission and etiology of this disease. By utilizing fecal samples, this PCR assay can be employed to track the presence of disease without sacrificing the animals, allowing transmission experiments to continue. Additionally, this PCR assay can be used retrospectively to investigate DNA samples from previous experiments or archives. Our studies highlight the value in taking a combined approach to studying the etiology of a disease. The research here provides the first step to understanding the cause and perhaps developing a controlled zebrafish model for intestinal cancer.
Supplementary Material
Supplemental Figure 1: Liver lesions. H&E. Bars = 50 μm A. Diffuse biliary hyperplasia, characterized by tortuous ducts with redundant profiles of approximately equal diameter lined by high cuboidal to low columnar epithelium and surrounded by prominent basement membranes. B. Higher magnifincation of A, X = inflammation (macrophage aggregates). C, D. Cholangiocellular carcinoma from a fish from Exposure D characterized by cellular atypia, poorly formed tubules, and invasion of normal liver parenchyma (arrows). Neoplastic exhibit indistinct cell margins with abundant granular eosinophilic cytoplasm. Nuclei are round to ovoid to fusiform with coarsely stippled chromatin and frequent prominent multiple nucleoli. Mitotic figures were but few, but there was prominent nuclear and cellular pleomorphism and atypia. Neoplastic cells frequently formed tortuous ducts of markedly variable diameter and shape. While these are generally lined by a single layer of neoplastic epithelial cells surrounded by prominent spindle-shaped cells, the epithelial cells often stack and form a pseudostratified appearance. Occasionally, nests of three or four neoplastic cells are observed in clusters that do not form ducts. The neoplasm infiltrative neoplasm frequently surrounded, separated, and entrapped clusters of hepatocytes.
Supplemental Table 1. Liver lesions in zebrafish exposed to fish with intestinal neoplasms.
Supplemental Text 1: Analysis of liver lesions.
Acknowledgments
We’d like to thank Poh Kheng Loi of the Histology and Genetic Modification (HGeM) Core Facility at the University of Oregon as well as Rose Sockol and Tiffani Jones for their contributions to the project. Research reported in this publication was supported by the National Institutes of Health under award numbers R01CA176579 (to KG and MK), R24OD010998 (to MK), ODNCRR P40 RR012546 (to MK), and P50GM098911 (to KG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
DR ADAM R BURNS (Orcid ID: 0000-0002-8402-598X)
References
- Anders K, Yoshimizu M. Role of Viruses in the induction of skin tumours and tumour-like proliferations of fish. Diseases of Aquatic Organisms. 1994;19:215–232. [Google Scholar]
- Barykova YA, Logunov DY, Shmarov MM, Vinarov AZ, Fiev DN, Vinarova NA, Gudkov AV. Association of Mycoplasma hominis infection with prostate cancer. Oncotarget. 2011;2(4):289–297. doi: 10.18632/oncotarget.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease. Cancer. 2001;91(4):854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bowser PR, Casey JW. Retroviruses of fish. Annual Review of Fish Diseases. 1993;3:209–224. [Google Scholar]
- Brennan CA, Garrett WS. Gut Microbiota, Inflammation, and Colorectal Cancer. Annual Review of Microbiology. 2016;70(1):395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard GW, Norris MB, Schwindt AR, Fournie JW, Winn RN, Kent ML, Ennis DG. Chronic Mycobacterium marinum infection acts as a tumor promoter in Japanese Medaka (Oryzias latipes) Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2009;149(2):152–160. doi: 10.1016/j.cbpc.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME Journal. 2012;6(8):1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesman SE, Neal JT, Mittge E, Seredick BM, Guillemin K. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd 88. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl(Supplement 1)):4570–7. doi: 10.1073/pnas.1000072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee LL, Casey JW, Bowser PR. Pathology of Tumors in Fish Associated With Retroviruses. Veterinary Pathology. 2013;50(3):390–403. doi: 10.1177/0300985813480529. [DOI] [PubMed] [Google Scholar]
- Dvir E, Clift SJ, Williams MC. Proposed histological progression of the Spirocerca lupi-induced oesophageal lesion in dogs. Veterinary Parasitology. 2010;168(1):71–77. doi: 10.1016/j.vetpar.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Feng SH, Tsai S, Rodriguez J, Lo SC. Mycoplasmal infections prevent apoptosis and induce malignant transformation of interleukin-3-dependent 32D hematopoietic cells. Molecular and Cellular Biology. 1999;19(12):7995–8002. doi: 10.1128/mcb.19.12.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba C, Guest S, Haywood S, Horney B. Chronic hepatitis: a retrospective study in 34 dogs. The Canadian Veterinary Journal. 1997;38(6):365–73. [PMC free article] [PubMed] [Google Scholar]
- Garrett WS. Cancer and the microbiota. Science. 2015;348(6230) doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell RG, Casey JW, Bowser PR. Seasonal Occurrence of Virally Induced Skin Tumors in Wild Fish. Journal of Aquatic Animal Health. 1998;10(2):191–201. [Google Scholar]
- Hamir AN, Smith BB. Severe biliary hyperplasia associated with liver fluke infection in an adult alpaca. Veterinary Pathology. 2002;39(5):592–594. doi: 10.1354/vp.39-5-592. [DOI] [PubMed] [Google Scholar]
- Hannon Lab. FastX Toolkit. 2010 Http://hannonlab.cshl.edu/fastx_toolkit/index.html.
- Holben WE, Williams P, Saarinen M, Särkilahti LK, Apajalahti JHA, Apajalahti JHA. Phylogenetic Analysis of Intestinal Microflora Indicates a Novel Mycoplasma Phylotype in Farmed and Wild Salmon. Microbial Ecology. 2002;44(2):175–185. doi: 10.1007/s00248-002-1011-6. [DOI] [PubMed] [Google Scholar]
- Hu B, Elinav E, Huber S, Strowig T, Hao L, Hafemann A, Flavell RA. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(24):9862–7. doi: 10.1073/pnas.1307575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Bishop-Stewart JK, Matthews JL, Spitsbergen JM. Pseudocapillaria tomentosa, a Nematode Pathogen, and Associated Neoplasms of Zebrafish (Danio rerio) Kept in Research Colonies. Comparative Medicine. 2002;52(4):354–358. [PubMed] [Google Scholar]
- Kent ML, Buchner C, Watral VG, Sanders JL, Ladu J, Peterson TS, Tanguay RL. Development and maintenance of a specific pathogen-free (SPF) zebrafish research facility for Pseudoloma neurophilia. Diseases of Aquatic Organisms. 2011;95(1):73–9. doi: 10.3354/dao02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Harper C, Wolf JC. Documented and Potential Research Impacts of Subclinical Diseases in Zebrafish. ILAR Journal. 2012;53(2):126–134. doi: 10.1093/ilar.53.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff H, Beyene P, Fischer M, Flossdorf J, Heitmann J, Khattab B, Yousef C. Mycoplasma mobile sp. nov., a New Species from Fish. International Journal of Systematic Bacteriology. 1987;37(3):192–197. [Google Scholar]
- Kirchhoff H, Rosengarten R. Isolation of a motile mycoplasma from fish. Journal of General Microbiology. 1984;130(9):2439–2445. doi: 10.1099/00221287-130-9-2439. http://doi.org/10.1099/00221287-130-9-2439. [DOI] [PubMed] [Google Scholar]
- Lane MC, Alteri CJ, Smith SN, Mobley HLT. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(42):16669–74. doi: 10.1073/pnas.0607898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn MS, McGinnity P, Dionne M, Letourneau J, Thonier F, Carvalho GR, Derome N. The biogeography of the atlantic salmon (Salmo salar) gut microbiome. The ISME Journal. 2016;10(5):1280–1284. doi: 10.1038/ismej.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nature Reviews Microbiology. 2014;12(10):661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti E, Gemei M, Mirabelli P, D’Alessio F, Di Noto R, Fortunato G, Del Vecchio L. The percentage of CD133+ cells in human colorectal cancer cell lines is influenced by Mycoplasma hyorhinis infection. BMC Cancer. 2010;10(1):120. doi: 10.1186/1471-2407-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M, Varga ZM. Anesthesia and Euthanasia in Zebrafish. ILAR Journal. 2012;53(2):192–204. doi: 10.1093/ilar.53.2.192. [DOI] [PubMed] [Google Scholar]
- McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An Emerging Cause of Sexually Transmitted Disease in Women. PLoS Pathogens. 2011;7(5):e1001324. doi: 10.1371/journal.ppat.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger MJ, Reinisch C, Sherry J, Goff SP. Horizontal Transmission of Clonal Cancer Cells Causes Leukemia in Soft-Shell Clams. Cell. 2015;161(2):255–263. doi: 10.1016/j.cell.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP. Clonally transmissible cancers in dogs and Tasmanian devils. Oncogene. 2008;27:S19–S30. doi: 10.1038/onc.2009.350. [DOI] [PubMed] [Google Scholar]
- Namiki K, Goodison S, Porvasnik S, Allan RW, Iczkowski KA, Urbanek C, Rosser CJ. Persistent Exposure to Mycoplasma Induces Malignant Transformation of Human Prostate Cells. PLoS ONE. 2009;4(9):e6872. doi: 10.1371/journal.pone.0006872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal JT, Peterson TS, Kent ML, Guillemin K. H. pylori virulence factor CagA increases intestinal cell proliferation by Wnt pathway activation in a transgenic zebrafish model. Disease Models & Mechanisms. 2013;6(3) doi: 10.1242/dmm.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Wagner H. vegan: Community Ecology Package 2016 [Google Scholar]
- Ostland VE, Watral V, Whipps CM, Austin FW, St-Hilaire S, Westerman ME, Kent ML. Biochemical, molecular, and virulence characteristics of select Mycobacterium marinum isolates in hybrid striped bass Morone chrysops × M. saxatilis and zebrafish Danio rerio. Diseases of Aquatic Organisms. 2007;79(2):107–118. doi: 10.3354/dao01891. [DOI] [PubMed] [Google Scholar]
- Paquette CE, Kent ML, Buchner C, Tanguay RL, Guillemin K, Mason TJ, Peterson TS. A Retrospective Study of the Prevalence and Classification of Intestinal Neoplasia in Zebrafish (Danio Rerio) Zebrafish. 2013;10(2):228–236. doi: 10.1089/zeb.2012.0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette CE, Kent ML, Peterson TS, Wang R, Dashwood RH, Löhr CV. Immunohistochemical characterization of intestinal neoplasia in zebrafish Danio rerio indicates epithelial origin. Diseases of Aquatic Organisms. 2015;116(3):191–7. doi: 10.3354/dao02924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek RM. New Biology to New Treatment of Helicobacter pylori-Induced Gastric Cancer. Digestive Diseases (Basel, Switzerland) 2016;34(5):510–6. doi: 10.1159/000445231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MR, Weidner N. Gastrointestinal neoplasia associated with bowel parasitosis: real or imaginary? Journal of Tropical Medicine. 2011;2011:234254. doi: 10.1155/2011/234254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2016. [Google Scholar]
- Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, Rawls JF. Evidence for a core gut microbiota in the zebrafish. The ISME Journal. 2011;5(10):1595–1608. doi: 10.1038/ismej.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MB, Rogers BM. Mycoplasma and cancer: in search of the link. Oncotarget. 2011;2(4):271–273. doi: 10.18632/oncotarget.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S. Interaction of Mycoplasmas With Host Cells. Physiological Reviews. 2003;83(2) doi: 10.1152/physrev.00030.2002. [DOI] [PubMed] [Google Scholar]
- Samaras V, Rafailidis PI, Mourtzoukou EG, Peppas G, Falagas ME. Chronic bacterial and parasitic infections and cancer: a review. The Journal of Infection in Developing Countries. 2010;4(5):267–281. doi: 10.3855/jidc.819. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Watral V, Kent ML. Microsporidiosis in Zebrafish Research Facilities. ILAR Journal. 2012;53(2):106–113. doi: 10.1093/ilar.53.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmale M. Experimental induction of neurofibromatosis in bicolor damselfish. Diseases of Aquatic Organisms. 1995;23(3):201–212. [Google Scholar]
- Schmale M, Gibbs P, Campbell C. A virus-like agent associated with neurofibromatosis in damselfish. Diseases of Aquatic Organisms. 2002;49(2):107–115. doi: 10.3354/dao049107. [DOI] [PubMed] [Google Scholar]
- Sears CL, Garrett WS. Microbes, Microbiota, and Colon Cancer. Cell Host & Microbe. 2014;15(3):317–328. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla L, Juyal P, Sandhu B. Pathology and therapy in naturally eimeria stiedae-infected rabbits. J Protozool Res. 2000;10:185–191. [Google Scholar]
- Spitsbergen JM, Tsai HW, Reddy A, Miller T, Arbogast D, Hendricks JD, Bailey GS. Neoplasia in Zebrafish (Danio rerio) Treated with 7,12-Diniethylbenz[a]anthracene by Two Exposure Routes at Different Developmental Stages. Toxicologic Pathology. 2000;28(5):705–715. doi: 10.1177/019262330002800511. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Buhler DR, Peterson TS. Neoplasia and neoplasm-associated lesions in laboratory colonies of zebrafish emphasizing key influences of diet and aquaculture system design. ILAR Journal. 2012;53(2):114–25. doi: 10.1093/ilar.53.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtläander CT, Lotz W, Körting W, Kirchhoff H. Piscine gill epithelial cell necrosis due to Mycoplasma mobile strain 163 K: comparison of in-vivo and in-vitro infection. Journal of Comparative Pathology. 1995;112(4):351–9. doi: 10.1016/s0021-9975(05)80016-7. [DOI] [PubMed] [Google Scholar]
- Stadtländer C, Kirchhoff H. Surface parasitism of the fish mycoplasma Mycoplasma mobile 163 K on tracheal epithelial cells. Veterinary Microbiology. 1990;21(4):339–343. doi: 10.1016/0378-1135(90)90005-g. [DOI] [PubMed] [Google Scholar]
- Stephens WZ, Burns AR, Stagaman K, Wong S, Rawls JF, Guillemin K, Bohannan BJM. The composition of the zebrafish intestinal microbial community varies across development. The ISME Journal. 2016;10(3):644–54. doi: 10.1038/ismej.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuppeveld FJ, Johansson KE, Galama JM, Kissing J, Bölske G, van der Logt JT, Melchers WJ. Detection of mycoplasma contamination in cell cultures by a mycoplasma group-specific PCR. Applied and Environmental Microbiology. 1994;60(1):149–52. doi: 10.1128/aem.60.1.149-152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clinical Microbiology Reviews. 2004;17(4):697–728. doi: 10.1128/CMR.17.4.697-728.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73(16):5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren A, Center S, McDonough S, Chiotti R, Goldstein R, Meseck E, Simpson K. Histopathologic features, immunophenotyping, clonality, and eubacterial fluorescence in situ hybridization in cats with lymphocytic cholangitis/cholangiohepatitis. Veterinary Pathology. 2011;48(3):627–641. doi: 10.1177/0300985810384409. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book, 5th Edition; A guide for the laboratory use of zebrafish (Danio rerio) Eugene, Oregon: University of Oregon Press; 2007. [Google Scholar]
- Whipps CM, Lieggi C, Wagner R. Mycobacteriosis in Zebrafish Colonies. ILAR Journal. 2012;53(2):95–105. doi: 10.1093/ilar.53.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewski LE, Peek RM, Coburn LA. The Role of the Microbiome in Gastrointestinal Cancer. Gastroenterology Clinics of North America. 2016;45(3):543–556. doi: 10.1016/j.gtc.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Qu L, Ma H, Chen L, Liu W, Liu C, Shou C. Mycoplasma hyorhinisinfection in gastric carcinoma and its effects on the malignant phenotypes of gastric cancer cells. BMC Gastroenterology. 2010;10(1):132. doi: 10.1186/1471-230X-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Liver lesions. H&E. Bars = 50 μm A. Diffuse biliary hyperplasia, characterized by tortuous ducts with redundant profiles of approximately equal diameter lined by high cuboidal to low columnar epithelium and surrounded by prominent basement membranes. B. Higher magnifincation of A, X = inflammation (macrophage aggregates). C, D. Cholangiocellular carcinoma from a fish from Exposure D characterized by cellular atypia, poorly formed tubules, and invasion of normal liver parenchyma (arrows). Neoplastic exhibit indistinct cell margins with abundant granular eosinophilic cytoplasm. Nuclei are round to ovoid to fusiform with coarsely stippled chromatin and frequent prominent multiple nucleoli. Mitotic figures were but few, but there was prominent nuclear and cellular pleomorphism and atypia. Neoplastic cells frequently formed tortuous ducts of markedly variable diameter and shape. While these are generally lined by a single layer of neoplastic epithelial cells surrounded by prominent spindle-shaped cells, the epithelial cells often stack and form a pseudostratified appearance. Occasionally, nests of three or four neoplastic cells are observed in clusters that do not form ducts. The neoplasm infiltrative neoplasm frequently surrounded, separated, and entrapped clusters of hepatocytes.
Supplemental Table 1. Liver lesions in zebrafish exposed to fish with intestinal neoplasms.
Supplemental Text 1: Analysis of liver lesions.


