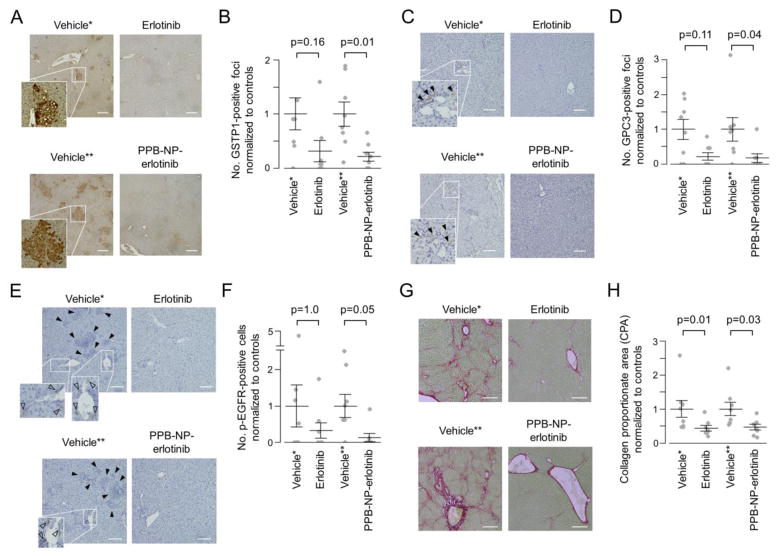
Figure 4.
In vivo chemopreventive effect of myofibroblast-targeted EGFR inhibition. (A) Immunohistochemistry of GSTP1 protein. Scale bar indicates 200 μm. (B) Number of GSTP1-positive foci per visual field normalized to respective controls according to the treatment groups (n=8 per group). Wilcoxon rank-sum test p-values (Bonferroni-corrected) are shown. Longer and shorter horizontal bars indicate mean and standard error of mean, respectively. (C) Immunohistochemistry of GPC3 protein. Closed arrow head indicates GPC3-positive focus. Scale bar indicates 200 μm. (D) Number of GPC3-positive foci per visual field normalized to respective controls according to the treatment groups (n=8 per group). (E) Immunohistochemistry of p-EGFR protein. Open arrow head indicates p-EGFR-positive cell. Closed arrow head indicates neoplastic foci with hyper-eosinophilic cytoplasm and GSTP1 positivity in serial tissue section as shown in panel A. Scale bar indicates 100 μm. (F) Number of p-EGFR-positive cells normalized to respective controls according to the treatment groups (n=8 per group). (G) Sirius red staining to visualize and quantify fibrotic tissue. Scale bar indicates 200 μm. (H) Collagen proportionate area (CPA) per visual field normalized to respective controls according to the treatment groups (n=8 per group).
*Vehicle control for systemic erlotinib administration (i.e., intraperitoneal injection). **Vehicle control for myofibroblast-targeting nanoparticles (i.e., PPB-NP). PPB: platelet-derived growth factor receptor-β-binding peptide, NP: nanoparticle.