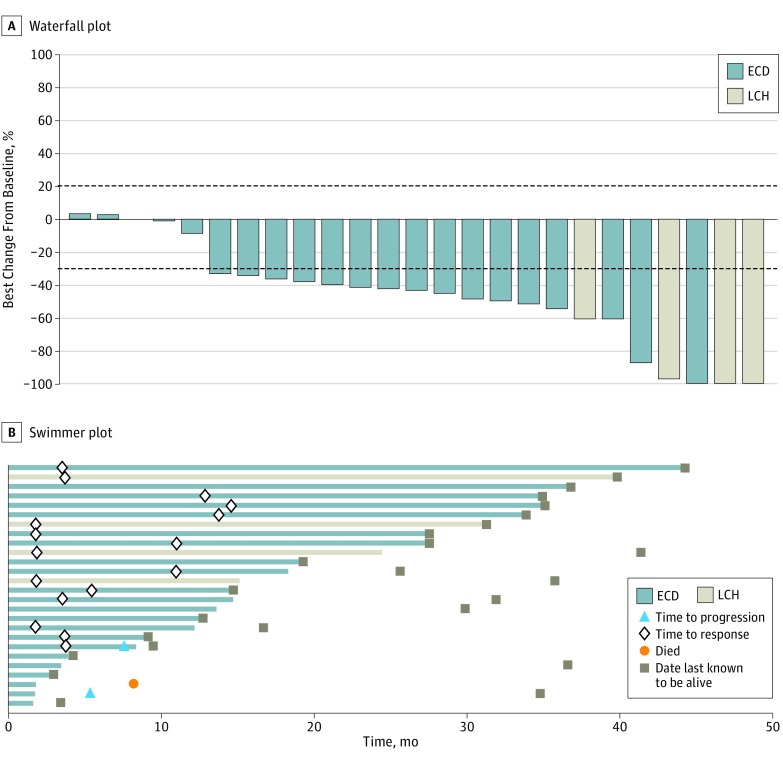
Figure 1. Efficacy of Vemurafenib in Individual Patients With BRAF V600–Mutant Erdheim-Chester Disease (ECD) or Langerhans Cell Histiocytosis (LCH).
One patient did not have measurable disease at baseline and was thus not evaluable for response but was included in the intention-to-treat analysis as a nonresponder. The line at −30% represents the cutoff for partial response by RECIST criteria (Response Evaluation Criteria in Solid Tumors); the line at +20% demarcates disease progression, which did not occur in any of the patients in this study.