Figure 4.
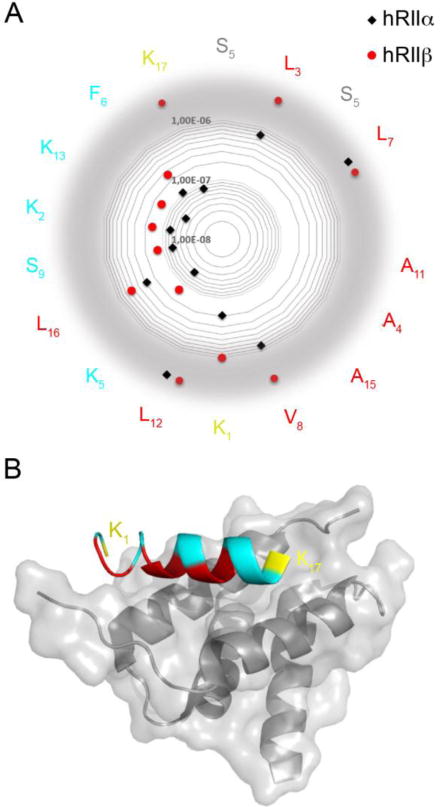
a) Representation of KD values on a radar plot of the STAD-2 peptide sequence. KD values for hRIIα are shown in black and hRIIβ are shown in red. The amino acids facing the D/D domain are depicted in red, the solvent exposed in teal. The hydrocarbon staple is illustrated in grey and the flanking lysines are yellow. While substitutions at many positions were largely inconsequential and indicate that they are not major contributors to the AKAP:PKA binding interface (K2, F6, S9, K13), substitutions at position K1 and K17 were better tolerated by hRIIα than by hRIIβ, indicating that these terminal residues may perform key contacts to confer isoform selectivity between RIIα and RIIβ. Values out of the scale are displayed on a gray border. b) Structural representation highlighting the terminal positions 1 and 17 (yellow) of an AKAP peptide bound to the D/D domain of RIIα. The critical hydrophobic residues that are important for binding to both RIIα and RIIβ are shown in red. The structure was rendered using PDB 2hwn.
