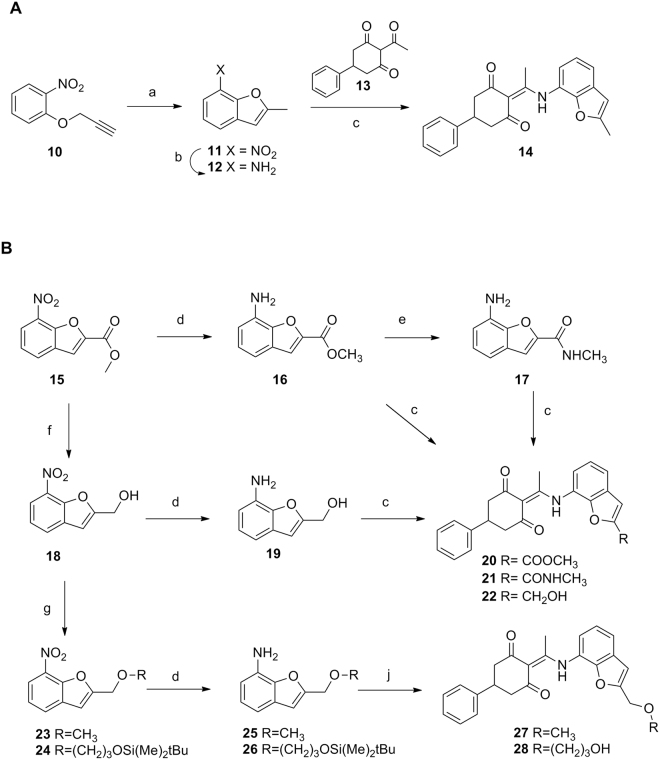
Figure 4.
Synthesis of the benzofurane derivatives. Reagents and conditions: (a) PEG-500, 215 °C, 90 min; (b) SnCl2, HCl, MeOH, 75 °C, 30 min; (c) 13, toluene, 4 Å molecular sieves, pressure tube, 110 °C, overnight. (d) H2, 5% Pt/S, AcOEt, 30 psi, rt, 2–8 h.; (e) NH2CH3, NH4Cl, MeOH, MW, 60 °C, 3 h; (f) DIBAL, Et2O, rt, 6 h; (g) 13, toluene, 4 Å molecular sieves, pressure tube, 110 °C, overnight. (h) For 23: MeI, NaH, anh. DMF, rt, 2 h; for 24: (3-bromopropoxy)(tert-butyl)dimethylsilane, 50% NaOH, TBABr, THF, 60 °C, 4 h; (j) for 27: 13, toluene, 4 Å molecular sieves, pressure tube, 110 °C, overnight; (j) for 28: (i) 13, toluene, 4 Å molecular sieves, pressure tube, 110 °C, overnight, (ii) TFA, CH2Cl2, rt, 1 h.