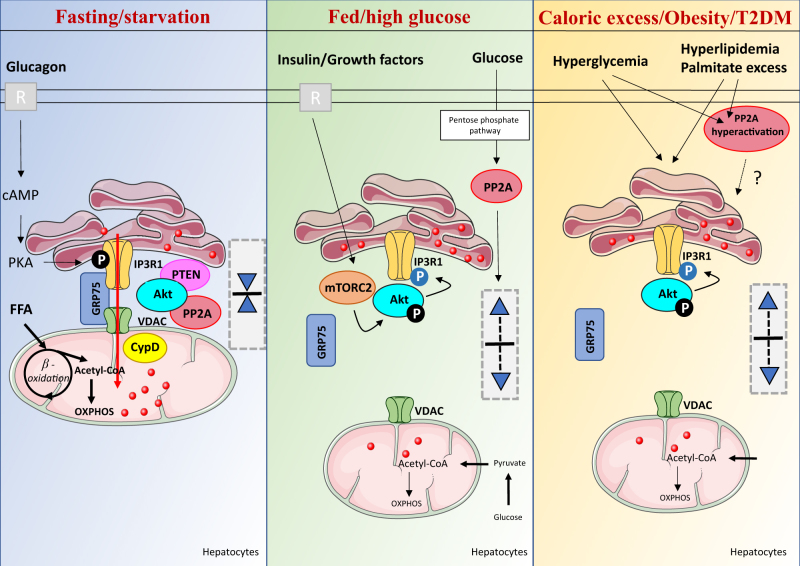
Fig. 3. Dynamic regulations of ER-mitochondria interactions in hepatocytes in function of nutritional and pathological states.
Left: At fasted or starved state, physical ER-mitochondria interactions are favored in order to increase oxidative capacities of mitochondria and preferentially use free fatty acids (FFA) as metabolic substrate. In this context, glucagon increases the phosphorylation and the activity of IP3R, leading to increased Ca2+ transfer from ER to mitochondria and to the stimulation of mitochondria metabolism. Both PP2A and PTEN probably counteract Akt-mediated reduction of IP3R-mediated Ca2+ release. Middle: At fed state or following high glucose stimulation, ER-mitochondria interactions and Ca2+ transfer are reduced by a mechanism dependent of PP2A. In this context, mTORC2 activate Akt phosphorylation, which induces the phosphorylation and inhibition of IP3R, leading to a reduction IP3R-mediated Ca2+ release. Upon caloric excess, increased glycemia or lipidemia reduce ER-mitochondria interactions. In this context, PP2A hyperactivation might participate to ER-mitochondria miscommunication associated with obesity and T2DM