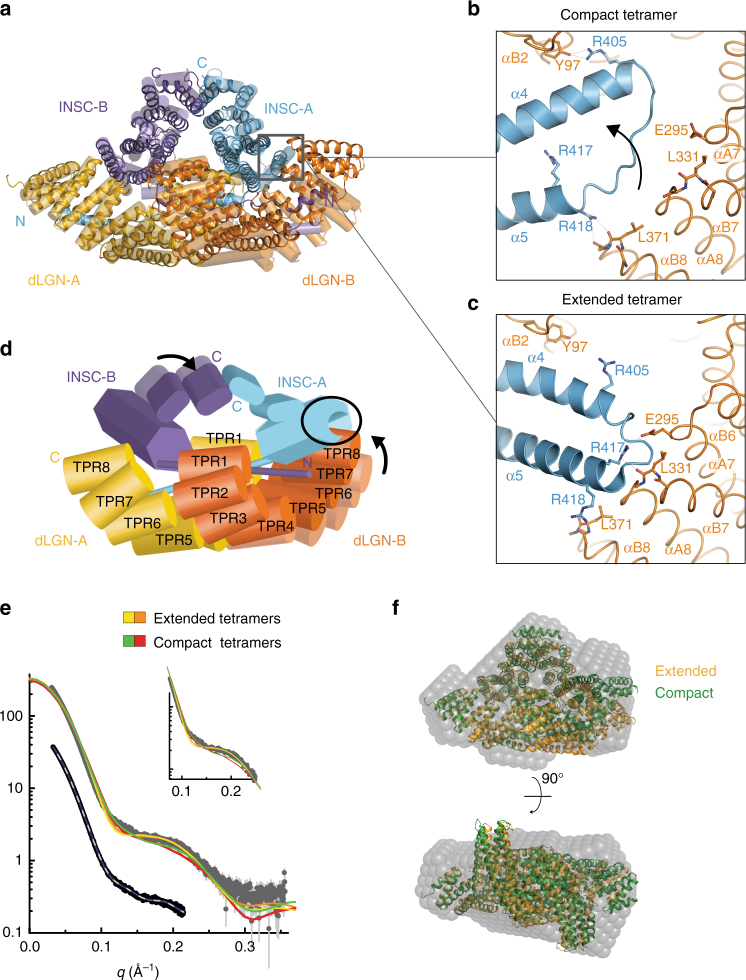
Fig. 2.
The dLGNTPR:InscASYM complex adopts two conformations. a Superposition of the two dLGNTPR:InscASYM conformers present in the a.s.u. The most compact conformer is shown as ribbon diagram, and the most extended is depicted in transparent cylindrical helices. Subunits are colored as in Fig. 1. b, c Zoomed views of the interfaces between the helical bundle of Insc-A and TPR7-8 of dLGN-B for the compact (top) and extended (bottom) tetramers. The rearrangement of the TPR repeats of dLGN-B in the compact conformation is accompanied by disruption of the initial turns of helix α5 of Insc-A. d Graphical summary of the conformational changes between the extended and the compact tetramers. e SAXS data for the dLGNTPR:InscASYM complex in comparison to the theoretical curves for the different configurations (upper part and inlay) and fits of ab initio models in P1 (gray) and P2 symmetry (blue). Idealized curves were obtained merging frames with matching radius of gyration and high similarity according to CORMAP38,39. f The ab initio model of the dLGNTPR:InscASYM complex in P1 symmetry (transparent gray spheres) matches well with both the extended (orange) and compact (green) tetramers