Fig. 3.
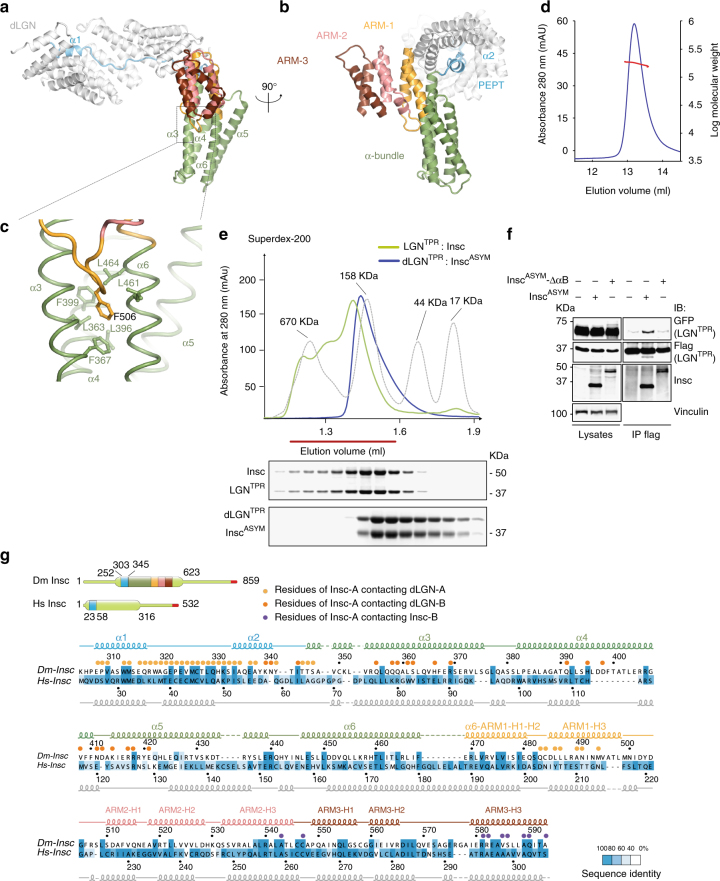
The topology of the asymmetric domain of Insc is evolutionary conserved. a, b Cartoon model of InscASYM bound to the inner groove of dLGNTPR (in white) at two orthogonal views. After the initial InscPEPT stretch (in cyan), the Insc chain departs from the TPR α-solenoid forming a four-helix bundle (green). The C-terminal part of the last helix of the bundle kinks into two short helical fragments of the first non-canonical ARM repeat (gold), which is followed by two conventional ARM motifs adopting a triangular flat shape (pink and brown). c Zoom of the hydrophobic pocket in which Phe506Insc fits. d Static-Light-Scattering profile of the dLGNTPR:InscASYM tetramer showing an average molecular mass of about 180 kDa along the peak, as expected for a 2:2 tetramer. e SEC elution profiles of Drosophila dLGNTPR:InscASYM (blue trace) and human LGNTPR:Insc (green trace) with associated Coomassie-stained SDS–PAGE separation of peak fractions. The elution profile of globular markers is reported in a dashed gray line. Both complexes elute in fractions consistent with the theoretical molecular mass of 2:2 tetramers. f InscASYM-ΔαB assembles with LGNTPR in a 1:1 stoichiometry. HEK293T cells were transiently transfected with plasmids containing human GFP-LGNTPR and FLAG-LGNTPR (residues 1–350) alone or in combination with human InscASYM or InscASYM-ΔαB (lacking residues 62–191). After 48 h, cells lysates were immunoprecipitated (IPs) with anti-FLAG antibodies conjugated to sepharose beads, and immunoblotted (IB) with the indicated antibodies. FLAG-LGNTPR- was able to co-immunoprecipitate with GFP-LGNTPR only when bound to InscASYM, but not to InscASYM-ΔαB. g Sequence alignment of InscASYM with secondary structure elements based on the crystallographic structure (for Drosophila) and in silico prediction (for Homo sapiens). Residues are colored according to the degree of conservation calculated on the basis of the alignment of Supplementary Fig. 4. Circles indicate residues of InscASYM in contact with the other three subunits of the tetramer