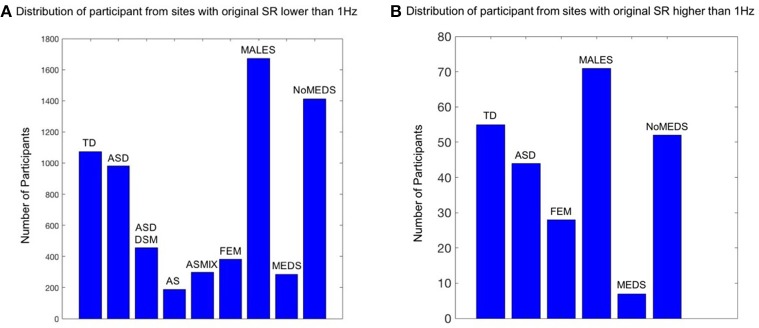
Figure 1.
Inclusion / Exclusion criteria for the ABIDE I and II data sets used in this study. Inclusion / Exclusion criteria for the ABIDE I and II data sets used in this study. (A) Participants from the sites from ABIDE I and ABIDE II which collected the fMRI data using a sample resolution lower than 1 Hz. TD refers to typically developing participants (SR0 n = 1074; SR1 n = 55); ASD refers to Autism Spectrum Disorders according to the column 1 DSM of demographic records across ABIDE I and II (SR0 n = 982; SR1 n = 44). ASDDSM indicates those participants from the column 2 of demographic records with a DSM-IV-TR ASD diagnosis (SR0 n = 456); AS refers to the individuals from DSM-IV-TR with Asperger's diagnosis of column 2 of demographics records (SR0 n = 189); ASMIX includes all AS, (Pervasive Developmental Disorder Not Otherwise Specified (PDDNOS), Pervasive Developmental Disorder (PDD) from column 2 DSM-IV (no ASD from DSM IV) (SR0 n = 298). FEM refers to all the female participants of demographic records across ABIDE I and II, despite the diagnosis they have (SR0 n = 384; SR1 n = 28); MALES are all male participants of demographics records, despite the diagnosis they have (SR0 n = 1673; SR1 n = 71); NoMEDS refers to all with a diagnosis of ASD (column 1 and 2 of the demographics records), all with a diagnosis of AS or PDDNOS or PDD who were not on medication (i.e., from all sites that reported medications) (SR0 n = 1414; SR1 n = 52). MEDS refers to all participants with any diagnosis but not on medication (SR0 n = 285; SR1 n = 7). (B) The same as (A) for participants from the sites which collected the fMRI data using a sample resolution higher than 1 Hz.