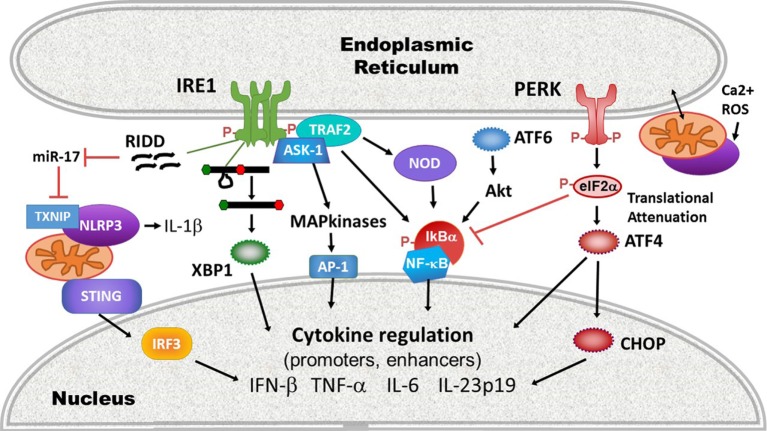
Figure 3.
Intersections of endoplasmic reticulum (ER) stress/unfolded protein response (UPR) and immune signaling. ER stress and the UPR impact innate immune signaling and cytokine production on many levels between pathogen-sensing pattern recognition receptors (PRRs) and ultimate cytokine production: (1) PRR activation: ER stress and the UPR activate multiple PRRs (purple) including stimulator of interferon gene (STING), NLRP3, and other inflammasomes via thioredoxin-interacting protein (TXNIP) upregulation and reactive oxygen species (ROS), and the NOD1/2 receptors. Much of this interaction occurs at the mitochondrial-ER interface, where released calcium (Ca2+) and ROS feed into PRR activation. (2) The UPR enhances inflammatory signaling pathways leading to mitogen-activated protein kinase activation [IRE1 shown here, but protein kinase R-like endoplasmic reticulum kinase (PERK) and activating transcription factor 6 (ATF6) also impact ERK and p38 activation], and inhibitory factor κB (IκB) phosphorylation and degradation. (3) Transcription factors: the UPR activates canonical pro-inflammatory and IFN-regulatory transcription factors activator protein 1 (AP-1), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and IRF3. Core UPR generated transcription factors such as XBP1 and C/EBP homologous protein (CHOP) also directly stimulate cytokine production by binding cytokine promoter and enhancer elements.