Abstract
Purpose
The aim of this study is to evaluate which cryopreservation protocol, freezing before or after swim-up, optimizes cryopreservation outcomes in terms of motile sperm count, motility, morphology, and viability, and also to establish whether sperm viability could be assessed based on sperm motility.
Methods
Fifty-three fresh and 53 swim-up prepared samples were considered for the first experiment. In parallel, total motility evaluation by CASA system (computer-assisted sperm analyzer) and hypoosmotic swelling test (HOS-test) was performed in each sample to compare the viability results of both methods. In the second experiment, 21 normozoospermic semen samples and 20 semen samples from male factor patients were included. After fresh ejaculate evaluation, the semen sample of each patient was divided into two aliquots, one of them was frozen before swim-up and the other was frozen after swim-up. Motility, sperm count, morphology, and viability were evaluated after thawing.
Results
A linear regression model allows prediction of HOS-test viability results based on total motility: HOS = 1.38 + 0.97 · TM (R 2 = 99.10, residual mean squares = 9.51). Freezing before sperm selection leads to higher total and progressive motility, total motile sperm count, and viability rates than when sperm selection is performed before freezing (P < 0.005 in all cases). In fact, sperm selection prior to freezing reaches critical values when subfertile patients are considered.
Conclusions
To conclude, total motility evaluation can predict HOS-test viability results, resulting in a more objective and less time-consuming method to assess viability. In addition, sperm freezing prior to swim-up selection must be considered in order to achieve better outcomes after thawing, especially in patients presenting poor sperm baseline.
Keywords: Total motile sperm count, Total motility, Sperm freezing, Seminal plasma, Swim-up, Viability
Introduction
In spite of all research done in the field of cryobiology including vitrification and rapid and programmable freezing, survival yields after sperm freezing are still low due to the damage to sperm cells, so optimizing sperm freezing protocol is a major concern due to semen cryopreservation is, currently, the only method in order to preserve male fertility.
Detrimental effects are a response to mechanical and osmotic damage due to temperature decrease and addition of cryoprotectant agents. Cryodamage affects membrane integrity, so sperm viability is affected by sperm freezing [1–4]. Viability of a semen sample can be evaluated by the hypoosmotic swelling test (HOS-test) or eosin test. These tests focus on spermatozoon membrane integrity, so non-motile but viable spermatozoa can be detected by these methods. However, these tests present some disadvantages that evince the need of a more objective and simpler method to assess sperm viability. Moreover, a decrease in sperm motility is observed in thawed samples, in part as a result of mitochondrial damage [5–7]. Sperm viability and motility are two parameters strongly correlated since spermatozoa presenting any type of motility are viable. In addition, mechanical and osmotic stress could affect sperm normal morphology [6, 8, 9]. Furthermore, during cryopreservation, there is an increase in reactive oxygen species (ROS) endogenous production that induces DNA fragmentation [10–12]. However, the effect of sperm freezing on DNA fragmentation remains controversial.
Before any artificial reproduction technique (ART), a sperm selection technique must be performed. Density gradient centrifugation (DGC) and swim-up are the two sperm preparation techniques most commonly used. In the literature, studies that choose either swim-up or DGC as a selection technique obtaining good results in both cases can be found [13–15]. The common point of these selection techniques is the fact that both remove seminal plasma present in fresh ejaculates and lead to a selection of a group of spermatozoa which exhibit better sperm features.
The protective role of seminal plasma during cryopreservation must be considered. Seminal plasma is present in fresh ejaculates and it is removed after sperm preparation. Antioxidants present in seminal plasma prevent ROS-induced damage during freezing. Moreover, polyunsaturated fatty acids (PUFAs) [16] or heparin-binding proteins (HBPs) [17] present in seminal plasma have a protective role during temperature decrease. Physical adsorption of certain proteins to spermatozoon surface could also prevent the thermal shock [18]. Hence, freezing fresh ejaculate before sperm preparation or selection could take advantage of the protective role of seminal plasma.
Even though several molecular markers are used in some studies to evaluate cryopreservation outcome, sperm count is one of the relevant factors to consider. Recent studies in testicular cancer patients’ cryopreservation [19], in ongoing pregnancy [20], and in ICSI cycles [21] suggest the relevance of total motile sperm count (TMSC) in achieving better reproductive outcomes since it is a parameter that combines sperm total motility and sperm concentration. TMSC gains importance when sample availability is limited and semen cryopreservation is indicated, such as in cases of pseudoazoospermia [22], in azoospermic patients who underwent surgical sperm retrieval, or in oncologic patients to guarantee future biological offspring after gonadotoxin treatments [23–26]. In this group of patients, it is vital to obtain the maximum number of motile (or viable) spermatozoa.
Therefore, the aim of this study is to compare which cryopreservation protocol, sperm cryopreservation before or after sperm preparation, leads to better outcomes. Also, the most suitable variables to evaluate cryopreservation outcomes are studied.
Material and methods
The current prospective study was held at the Andrology Laboratories of Assisted Reproduction Unit at Hospital La Fe (Valencia, Spain) and was approved by the Ethical Committee. Informed consent was obtained from all individual participants included in the study. All semen samples were collected by masturbation after an abstinence period of 3 to 5 days and were kept in sterile containers. Semen samples were processed 1 h after they arrived at the Andrology Laboratory. All procedures included in this research work as semen preparation, cryopreservation, sperm count, motility, morphology, and viability evaluation were performed using 1 ml of surplus semen samples after semen diagnosis assessment. Exclusion criteria included ejaculate volume less than 3 ml, post-vasectomy controls, currently active sexual transmitted disease (HIV, hepatitis B virus, and hepatitis C virus) samples, and semen collected by surgical retrieval techniques.
Semen samples from 106 male patients who attended the Andrology Laboratory were included in the first sample set. In this sample series were considered fresh ejaculates (53 samples) as well as prepared samples by swim-up (53 samples). Viability assessed by HOS-test and total sperm motility evaluated automatically were determined in each sample. The purpose of this study was to establish a relationship between viability and percentage of total motile spermatozoa.
A second sample set comprised semen samples from 41 men undergoing fertility diagnosis evaluation. Study series was divided into two groups according to semen diagnosis results: one group included 21 normozoospermic samples (according to WHO 2010 criteria), whereas the second group included 20 semen samples presenting abnormal sperm count and/or progressive motility according to WHO 2010 criteria. Hence, in this study, the group of patients presenting male factor subfertility refers to oligo-, astheno-, and oligoasthenozoospermic patients.
Sperm count (SC), progressive (PM) and total sperm motility (TM), and morphology (M) were evaluated automatically using a semen analyzer. Then, semen samples were divided into two aliquots (0.5 ml per aliquot). While one aliquot was prepared by swim-up technique prior to cryopreservation, the other aliquot was cryopreserved and then was prepared by swim-up technique after thawing.
Viability assay: HOS-test
HOS-test solution was prepared by diluting 0.735 g of dihydrated sodium citrate (SIGMA Aldrich, USA) and 1.351 g of D-fructose (SIGMA Aldrich, USA) in a total volume of 100 ml of ultrapure water. HOS solution was divided in 1-ml aliquots. Then, the aliquots were frozen at − 20 °C until their use.
According to WHO 2010 recommendations, thawed HOS-test solution aliquot was well mixed with 100 μl of the semen sample. After the incubation period (37 °C, 30 min), a 10-μl drop was placed in a microscope slide (22 × 22 mm) (Knittel Glass, Germany). A total of 200 spermatozoa were counted at ×400. The same operator performed the counting twice in each sample. Results are the average value. There were no interobserver variations due to all the evaluations were done by the same operator in order to avoid variations. Viable spermatozoa show a swelled tail. Viability percentage was obtained considering the number of spermatozoa with swelled tails out of 200 spermatozoa counted.
Sperm count and motility evaluation
Sperm count and motility were evaluated automatically using ISAS® software (version 1.2; Serial Number 0030149D), which is a computer-assisted semen analyzer (CASA) system, coupled to a phase contrast microscope. This sort of analyzer avoids subjectivity. Samples are visualized under ×10 microscope objective. In order to obtain reliable results, each measure consisted an average value of three different captured fields.
Morphology analysis
Sperm morphology assessment requires a stained sample. Diff Quick was the staining method chosen for sperm morphology evaluation. First, the semen sample extended in a microscope slide (22 × 22 mm) (Knittel Glass, Germany) is incubated in absolute ethanol (Panreac AppliChem ITW Reagents, Germany) for 30 s; once the slide is dried, it is incubated with eosin (Quick Panoptic Nr.2, QCA, Spain) and eosin blue (May-Grünwald’s eosine-methylene blue solution modified, Merck, Germany), respectively, for 30 s each. Finally, surplus of staining reactants is washed with distilled water. After the final drying step, the slide is covered and fixed to a cover slip using EUKIT®.
The stained slide is analyzed automatically by ISAS® software (version 1.2; Serial Number 0030149D) coupled to a phase contrast microscope. The sample is visualized under ×100 microscope objective and 100 spermatozoa are captured per measure. The ISAS® software allows the evaluation of head, acrosome, and midpiece morphology.
Swim-up preparation prior to cryopreservation (SW-F)
In order to perform swim-up preparation technique, 0.5 ml aliquot of fresh ejaculate was mixed with washing medium (Flushing Medium, Irvine Scientific, USA) volume to volume (v/v) before a centrifugation step (320g, 10 min). The supernatant was discarded, and the pellet obtained after centrifugation was incubated with 100 μl of culture medium (IVF medium, Irvine Scientific, USA) for 45 min at 37 °C, 5% CO2 in the incubator (Labotect C200, Labor-Technik-Göttingen, Germany). Then, the upper phase of the culture medium (IVF medium, Irvine Scientific) containing motile spermatozoa was collected without disturbing the pellet. After the sample preparation step, both sperm count and motility (PM and TM) were evaluated automatically by the CASA system.
Then, the prepared sample was cryopreserved following slow freezing protocol. Before sperm freezing, 0.9 ml of the cryoprotectant agent (Test Yolk Buffer, TYB, Irvine Scientific, USA) was progressively added to 0.1 ml of the prepared sample. The semen sample was homogenized with TYB (Irvine Scientific, USA) and loaded in two 0.5-ml straws. Straws were kept at 4 °C for 20 min following a liquid nitrogen (LN) vapor step for 20 min before immersion in LN tank.
The thawing process was performed by incubating frozen straws at 37 °C, 5% CO2 for 15 min. The thawed sample was centrifuged (320g, 10 min) in order to remove the cryoprotectant. The pellet obtained was mixed with 0.1 ml of the culture medium (IVF, Irvine Scientific, USA). Finally, sperm count, TM, PM, and sperm morphology were evaluated.
Sperm cryopreservation before swim-up preparation (F-SW)
Firstly, 0.5 ml aliquot of fresh ejaculate was mixed progressively with 0.5 ml of cryoprotectant agent (Test Yolk Buffer, TYB, Irvine Scientific, USA) in order to cryopreserve the semen sample prior to swim-up preparation. Cryopreservation was performed following the slow freezing and thawing protocols described above.
Before the centrifugation step to eliminate the cryoprotectant, sperm count, PM, and TM evaluated automatically using the CASA system were recorded. After the centrifugation step (320g, 10 min), the supernatant was discarded and the pellet obtained was incubated with 100 μl of culture medium (IVF, Irvine Scientific, USA) in order to perform the swim-up technique as described above. Once sperm preparation was done, sperm count, PM, TM, and sperm morphology were evaluated automatically using the CASA system.
Statistical analysis
Results were analyzed using the software Statgraphics Centurion (version XVI.II). Results are displayed as mean value ± SD (standard deviation). Multivariate linear regressions were performed in order to build the viability prediction model based on the motility results. Linear regressions performed included TM, PM, and no PM (NPM), respectively, as well as sample origin (fresh or prepared sample). R 2 and residual mean squares (RMS) were used as accuracy indicators in order to compare models. Moreover, a comparison of RMS of the models was made using F test. t tests of dependent samples were performed in order to compare cryopreservation outcomes after both protocols were studied. P values < 0.05 were considered significant.
Results and discussion
Viability prediction based on total motility results
HOS-test viability and sperm motility were evaluated in the first sample set, in which 53 fresh samples and 53 prepared samples from different patients were included. The results obtained are displayed in Table 1. PM, NPM, TM, and HOS results comprised a wide range of values. Maximum and minimum values were the following: PM (0 to 99.30%), NPM (0 to 36.10%), TM (1.50 to 99.30%), and HOS (1.10 to 98%).
Table 1.
Motility and viability results
| Fresh | Prepared by swim-up | All (fresh + prepared) | |
|---|---|---|---|
| Number of samples | 53 | 53 | 106 |
| PM (a + b) ± SD | 33.90 ± 28.27 | 68.11 ± 30.50 | 51.00 ± 33.94 |
| NPM (c) ± SD | 6.86 ± 7.06 | 3.50 ± 5.65 | 5.18 ± 6.59 |
| TM ± SD | 40.76 ± 29.24 | 71.61 ± 29.39 | 56.18 ± 33.04 |
| HOS ± SD | 40.05 ± 39.37 | 70.79 ± 28.63 | 55.42 ± 31.87 |
PM %progressive motility, NPM %no progressive motility, TM %total motility, HOS percentage of viable spermatozoa after HOS-test, SD standard deviation
As is observed in Table 1, PM and TM are very close to HOS-test viability results. Multiple linear regression analyses were performed considering HOS-test results, sample origin, and PM and TM, respectively. In both cases, when PM and TM were considered, a relationship was found between sperm motility and viability. Here, the sample origin (fresh or prepared by swim-up) showed no effect (see Table 2). R 2 and RMS were used as accuracy indicators for the models.
Table 2.
Models for sperm viability prediction based on TM and PM
| Total motility (TM) | Progressive motility (PM) |
|---|---|
| HOS = 1.38 + 0.97 · TM | HOS = 8.56 + 0.93 · PM |
| R 2 | R 2 |
| 99.10 | 95.79 |
| RMS | RMS |
| 9.51 | 44.32 |
HOS percentage of viable spermatozoa after HOS-test, PM %progressive motility, TM %total motility, RMS residual mean square
TM predicts viability results more accurately than PM due to the differences of RMS between models considering PM and TM (44.32 vs 9.51) were not random according to F test result (P = 9.5 × 10−9).
In addition, a similar analysis was performed considering NPM. Interestingly, when NPM was considered in this multiple linear regression, also the sample origin (fresh or prepared) and the interaction of both explanatory variables were included in the model. Despite the poor accuracy (R2 = 29.31%) of the model, it is confirmed that NPM partly contributes to explain viability results demonstrating that no-progressive spermatozoa are also viable. As in this occasion, sample origin is a significant explanatory variable, represented in Table 3 by the models obtained for viability prediction based on NPM when fresh and prepared samples, separately, in order to clarify the expression of the results.
Table 3.
Viability prediction model based on NPM in fresh and prepared samples
| NPM in fresh samples | NPM in prepared samples |
|---|---|
| HOS = 31.48 + 1.11 · NPM | HOS = 73.92 − 0.58 · NPM |
| R 2 | R 2 |
| 8.23 | 1.38 |
| RMS | RMS |
| 713.66 | 806.09 |
HOS percentage of viable spermatozoa after HOS-test, NPM % no progressive motility, RMS residual mean square
Viability prediction based on sperm motility was designed as a preliminary experiment in order to establish whether HOS-test, one of the most common viability tests used in andrology, could be substituted for motility evaluation in order to assess sperm viability. In the literature reviewed, there are studies that call into question HOS-test reliability. Martini and colleagues demonstrate false positives after HOS-test [27]. In this study, they compared HOS-test and eosin test results. False positives after HOS-test are due to spontaneously developed tail swellings (SDTS) that occur when spermatozoa swelled after exposure to physiologic solution; so, in these cases, tail swelling is not a consequence of osmotic balancing. Spermatozoa that exhibit SDTS are not categorized as viable according to eosin staining in the cited study. Moreover, Hossain and collaborators also observe SDTS phenomenon under physiological conditions [3], as Martini reported recently [27]. In addition, Hossain and colleagues reported that the presence of SDTS was more frequent in cryopreserved samples [3]. Therefore, the development of a faster, simpler, economical, and more objective method for viability evaluation would be an interesting improvement.
TM is a sperm parameter that strongly correlates with viability results after HOS-test, as has been demonstrated in this experiment. TM has no negative impact on the sperm samples and allows the use of the semen sample in a following ART. Even though eosin staining would be another possible option in order to evaluate viability, it has some disadvantages such as less objectivity level because TM is evaluated by the CASA system and more time-consuming than TM evaluation, and it should be noted that eosin staining prevents use of stained sample in following ARTs. However, further research is needed in order to establish correlations between the eosin test and TM assessment.
Freezing before or after sperm preparation
A total of 41 semen samples from different patients aged from 20 to 41 years old were included in this experiment, 21 of them were from normozoospermic patients and 20 of them were from male factor patients.
Results of macroscopic and microscopic semen parameters of fresh ejaculates are summarized in Table 4.
Table 4.
Macroscopic and microscopic sperm parameters in fresh samples
| Normozoospermic patients | Male factor patients | All | P value | |
|---|---|---|---|---|
| Number of samples ± SD | 21 | 20 | 41 | |
| Volume (ml) ± SD | 4.34 ± 1.29 | 4.8 ± 1.48 | 4.56 ± 1.38 | 0.364 |
| pH ± SD | 8.30 ± 0.25 | 8.29 ± 0.12 | 8.3 ± 0.2 | 0.537 |
| Semen liquefaction ± SD | 2.88 ± 0.33 | 2.8 ± 0.4 | 2.84 ± 0.37 | 0.670 |
| Semen concentration ± SD | 101.69 ± 70.35 | 46.38 ± 36.35 | 67.36 ± 41.41 | 0.003 |
| PM ± SD | 59.66 ± 16.80 | 17.77 ± 9.02 | 39.23 ± 25.08 | < 0.001 |
| TM ± SD | 66.30 ± 16.51 | 27.07 ± 18.42 | 47.17 ± 26.30 | < 0.001 |
| PMSC ± SD | 60.09 ± 45.99 | 8.94 ± 8.28 | 35.14 ± 41.96 | < 0.001 |
| TMSC ± SD | 66.87 ± 48.53 | 13.30 ± 13.63 | 40.74 ± 44.73 | < 0.001 |
| Morphology ± SD | 4.50 ± 3.07 | 3.51 ± 1.67 | 4.02 ± 2.50 | 0.211 |
PM %progressive motility, TM %total motility, PMSC progressive motile sperm count (×106 spermatozoa/ml), TMSC total motile sperm count (×106 spermatozoa/ml), SD standard deviation
As it was expected, significant differences were observed in concentration, motility, and sperm count among normozoospermic and male factor patients (P < 0.001) (see Table 4).
Motility and sperm count after cryopreservation
Results of PM, TM, PMSC, and TMSC after both cryopreservation protocols considering all patients together are displayed in Table 5.
Table 5.
Microscopic sperm parameters after cryopreservation
| Preparation by swim-up prior cryopreservation | Cryopreservation prior swim-up preparation | P value | |
|---|---|---|---|
| Number of samples | 41 (normozoospermic and male factor) | ||
| Semen concentration ± SD | 16.75 ± 10.92 | 10.92 ± 11.71 | 0.052 |
| PM ± SD | 7.64 ± 7.55 | 37.38 ± 29.73 | < 0.001 |
| TM ± SD | 13.97 ± 11.75 | 38.71 ± 29.73 | < 0.001 |
| PMSC ± SD | 1.61 ± 2.60 | 5.41 ± 7.50 | < 0.001 |
| TMSC ± SD | 2.55 ± 3.17 | 5.62 ± 7.65 | 0.004 |
| Morphology | 3.06 ± 3.69 | 3.23 ± 3.42 | 0.079a |
| Viability ± SD | 14.93 ± 11.40 | 38.9 ± 28.84 | < 0.001 |
PM %progressive motility, TM %total motility, PMSC progressive motile sperm count (×106 spermatozoa/ml), TMSC total motile sperm count (×106 spermatozoa/ml), SD standard deviation
aMorphology P value refers to the comparison of normal morphology in fresh ejaculate and after sperm preparation prior to cryopreservation protocol
A significant decrease in all of the parameters evaluated is observed after sperm preparation by swim-up prior sperm freezing. Thus, sperm freezing before preparation by swim-up offers better cryopreservation outcomes in terms of motility and sperm count.
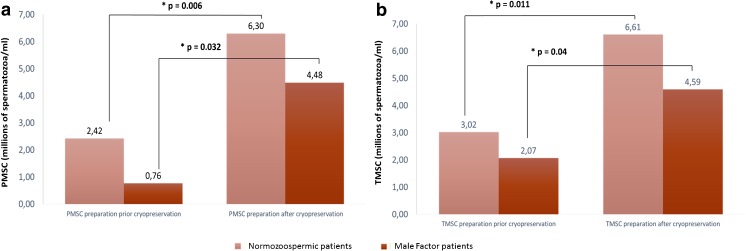
Significant differences are not only observed when all patients are considered, but when considering normozoospermic or male factor patients, differences are also still observed. Cryopreservation prior sperm preparation by swim-up results in higher PMSC and TMSC than when sperm preparation is performed prior sperm freezing in both groups of patients. In addition, considering the male factor group, PMSC and TMSC reach critical values (0.76 ± 0.90 and 2.07 ± 2.14) when sperm preparation prior cryopreservation is performed (Fig. 1). Since TMSC and PMSC give information about the number of motile spermatozoa obtained, achieving a low number of motile spermatozoa could affect the outcome of the following ART.
Fig. 1.
PMSC and TMSC after both cryopreservation protocols in normozoospermic and male factor patients. a PMSC, progressive motile sperm count (×106 spermatozoa/ml). b TMSC, total motile sperm count (×106 spermatozoa/ml). SD, standard deviation
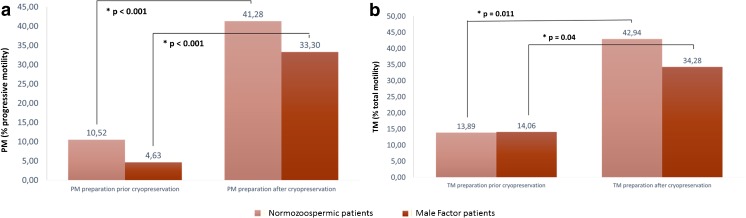
The same tendency is observed when TM and PM are analyzed, as shown in Fig. 2. Sperm preparation by swim-up prior cryopreservation impairs post-thawing PM and TM in both groups of patients considered in this study.
Fig. 2.
PM and TM after both cryopreservation protocols in normozoospermic and male factor patients. a PM, %progressive motility. b TM, %total motility. SD, standard deviation
Morphology analysis after cryopreservation
Table 5 displays morphology analysis results for both protocols. The results make reference to percentage of spermatozoa presenting normal morphology. A light increase in the percentage of spermatozoa with normal morphology is observed after cryopreservation before sperm preparation if it is compared with the other protocol.
According to the percentage of normal morphology forms in the fresh samples (4.02 ± 2.50), there is a tendency of decreased normal sperm cells after preparation by swim-up before cryopreservation (P = 0.079), but it lacks statistical significance.
Viability analysis after cryopreservation
Viability assessment before and after both cryopreservation protocols considered was performed applying the prediction model based on sperm total motility established previously in this study (HOS = 1.38 + 0.97 · TM) due to its strong correlation with HOS-test viability results. Viability results after both cryopreservation protocols are displayed in Table 5.
The results presented in Table 5 demonstrate that cryopreservation prior to sperm preparation leads to higher percentage of viable spermatozoa after thawing, since significant differences are observed in viability results among the two protocols.
Therefore, these results suggest that sperm preparation prior freezing impairs sperm viability after thawing, while sperm cryopreservation prior to sperm preparation achieves similar viability values to those observed in fresh samples.
There is need of optimizing sperm freezing protocols due to the poor survival rates in thawed samples. The present study demonstrates that significant differences are observed in terms of motility, motile sperm count, morphology, and viability depending on the protocol used. Our data show that sperm preparation prior to cryopreservation impairs semen sample quality after thawing, whereas cryopreservation before sperm preparation leads to higher TM, TMSC, PM, PMSC, and viability. Moreover, cryopreservation before sperm preparation has no impact on sperm morphology.
Even though it can be suggested that the poorer results in terms of motility and motile sperm count observed are due to the different dilutions used when mixing semen samples and TYB, it is worth to remark that during slow freezing, the cryodamage is mainly caused by ice crystal formation. Moreover, as described by Nallella and colleagues [28], TYB is the less toxic of the cryoprotectants compared in the cited study. However, as all aspects concerning sperm cryopreservation optimization are relevant, our group is working now on further studies in which potential toxicity of several cryoprotectants, including TYB, are being tested.
Drawing attention to other possible reasons, the odds are that the improvement observed after cryopreservation of fresh ejaculates before sperm preparation, when compared with sperm preparation prior to cryopreservation, can be due to the protective function of seminal plasma during cryopreservation. Several studies suggest that components of seminal plasma aid cryoresistance during temperature decrease [16–18]. Donnelly and colleagues also reported higher PM percentages when cryopreservation was performed before sperm preparation [29]. In addition, this study reinforces the protective role of seminal plasma during cryopreservation. Unfortunately, even though the CASA system was also used by Donnelly and colleagues, in their study, other motility or sperm count parameters are not evaluated. We defend that TM and TMSC are the two parameters to consider, due to TM is more correlated than PM with sperm viability as has been demonstrated in the present study. TMSC combines TM and sperm concentration, so it provides information about the amount of total motile spermatozoa available. This fact is especially relevant when sample availability is limited, and then the amount of spermatozoa available is a critical fact to consider.
However, other studies suggest that sperm preparation before cryopreservation raises higher motility percentages. Studies done by Petyim [30] and Esteves [31] suggest an improvement in motion characteristics when sperm preparation by swim-up is performed prior to cryopreservation. Regarding the study of Petyim, the results reported a slight improvement in sperm motility after freezing when the semen sample is prepared by swim-up (99.5% PM vs 93.9% PM). Despite the light difference in progressive motility percentage between the two cryopreservation protocols, it raises a high level of significance, possibly because of the low variability in motility values among the patients studied (from 41.9 to 64.2%) [30]. A comparison of these results with those obtained from a subpopulation of patients with asthenozoospermia, where, undeniably, the motility values will vary among the patients, would have been interesting. The results published by Esteves and collaborators show the same tendency [31]. Even though, in the cited research work, a slight improvement in sperm motility is reported after freezing when the semen sample is prepared by swim-up (30.1% PM vs 28% PM), it does not reach statistical significance, as is clearly shown in the result table provided by the authors. Therefore, the improvement in sperm motility is not clearly established.
However, according to the number of spermatozoa obtained in both studies, not only the total sperm count, but also the total motile sperm count is higher when fresh ejaculate is frozen prior to sperm selection [30, 31]; so, in terms of TMSC, their results agree with ours. Despite its high level of significance, this result is not detailed in-depth in the results or discussion. As TMSC provides information about the number of viable spermatozoa available, it should not be dismissed because it could guide to an inaccurate vision of the research performed.
Another relevant fact to remark is that these studies only include normozoospermic donors. TMSC gains importance when a population of subfertile men is considered due to these patients often present impaired sperm baseline. In this group of patients, it is vital to obtain the maximum number of motile spermatozoa. In the present study, it is demonstrated that when male factor group is considered isolated, critical values are obtained not only for TMSC but also for PMSC when sperm selection is performed before cryopreservation. Hence, cryopreservation prior to sperm selection achieves a higher number of total and progressive motile spermatozoa; that is why this protocol must be specially recommended in male factor patients who present limited sample availability.
A more recent study published by Brugnon in 2013 [32] studied a population of oligoasthenoteratozoospermia male patients, but in this case the study design is not as accurate as it could be since each study group includes different patients, so the same semen samples are not used for each cryopreservation protocol.
Conclusions
Sperm total motility strongly correlates with sperm viability; therefore, viability evaluation based on sperm total motility is a more objective, simpler, and less time-consuming method to assess sperm viability.
Sperm cryopreservation prior to sperm selection by swim-up leads to better cryopreservation outcomes in terms of PM, TM, PMSC, TMSC, and viability than sperm selection prior to cryopreservation. In contrast, the protocols considered do not affect sperm morphology. Regarding male factor group, sperm selection prior to cryopreservation achieves critical values of not only PM and TM, but also PMSC and TMSC. Therefore, it is demonstrated that sperm preparation after freezing should be considered to increase the available sperm number, especially in patients with poor sperm baseline undergoing repeated ICSI cycles.
Funding
No funding was received.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of Hospital La Fe Research Foundation (Instituto de Investigación Sanitaria La Fe) research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.Hammadeh ME, Dehn C, Hippach M, Zeginiadou T, Stieber M, Georg T, et al. Comparison between computerized slow-stage and static liquid nitrogen vapour freezing methods with respect to the deleterious effect on chromatin and morphology of spermatozoa from fertile and subfertile men. Int J Androl. 2001;24:66–72. doi: 10.1046/j.1365-2605.2001.00270.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhu WJ, Liu XG. Cryodamage to plasma membrane integrity in head and tail regions of human sperm. Asian J Androl China. 2000;2:135–138. [PubMed] [Google Scholar]
- 3.Hossain A, Osuamkpe C, Hossain S, Phelps JY. Spontaneously developed tail swellings (SDTS) influence the accuracy of the hypo-osmotic swelling test (HOS-test) in determining membrane integrity and viability of human spermatozoa. J Assist Reprod Genet. 2010;27:83–86. doi: 10.1007/s10815-009-9375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin MH, Morshedi M, Srisombut C, Nassar A, Oehninger S. Plasma membrane integrity of cryopreserved human sperm: an investigation of the results of the hypoosmotic swelling test, the water test, and eosin-Y staining. Fertil Steril. 1998;70:1148–1155. doi: 10.1016/S0015-0282(98)00351-3. [DOI] [PubMed] [Google Scholar]
- 5.O’Connell M, McClure N, Lewis SEM. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod. 2002;17:704–709. doi: 10.1093/humrep/17.3.704. [DOI] [PubMed] [Google Scholar]
- 6.Satirapod C, Treetampinich C, Weerakiet S, Wongkularb A, Rattanasiri S, Choktanasiri W. Comparison of cryopreserved human sperm from solid surface vitrification and standard vapor freezing method: on motility, morphology, vitality and DNA integrity. Andrologia. 2012;44(Suppl 1):786–790. doi: 10.1111/j.1439-0272.2011.01267.x. [DOI] [PubMed] [Google Scholar]
- 7.Oberoi B, Kumar S, Talwar P. Study of human sperm motility post cryopreservation. Med J Armed Forces India. 2014;70:349–353. doi: 10.1016/j.mjafi.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agha-Rahimi A, Khalili MA, Nabi A, Ashourzadeh S. Vitrification is not superior to rapid freezing of normozoospermic spermatozoa: effects on sperm parameters, DNA fragmentation and hyaluronan binding. Reprod BioMed Online. 2014;28:352–358. doi: 10.1016/j.rbmo.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Ozkavukcu S, Erdemli E, Isik A, Oztuna D, Karahuseyinoglu S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assist Reprod Genet. 2008;25:403–411. doi: 10.1007/s10815-008-9232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopeika J, Thornhill A, Khalaf Y. The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update. 2015;21:209–227. doi: 10.1093/humupd/dmu063. [DOI] [PubMed] [Google Scholar]
- 11.Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang JA, Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod. 2009;24:2061–2070. doi: 10.1093/humrep/dep214. [DOI] [PubMed] [Google Scholar]
- 12.Ribas-Maynou J, Fernández-Encinas a., García-Peiró a., Prada E, Abad C, Amengual MJ, et al. Human semen cryopreservation: a sperm DNA fragmentation study with alkaline and neutral comet assay. Andrology 2014;2:83–87. [DOI] [PubMed]
- 13.Michaeli M, Peer S, Anderman S, Ballas S, Ellenbogen A. Post swim-up versus original sperm quality, and strict criteria morphology, it’s influence on fertilization rate in in vitro fertilization program: a pilot study. Int Congr Ser. 2004;1271:181–184. doi: 10.1016/j.ics.2004.07.002. [DOI] [Google Scholar]
- 14.Ricci G, Perticarari S, Boscolo R, Montico M, Guaschino S, Presani G. Semen preparation methods and sperm apoptosis: swim-up versus gradient-density centrifugation technique. Fertil Steril. 2009;91:632–638. doi: 10.1016/j.fertnstert.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 15.Fácio CL, Previato LF, Machado-Paula LA, Matheus PCS, Araújo FE. Comparison of two sperm processing techniques for low complexity assisted fertilization: sperm washing followed by swim-up and discontinuous density gradient centrifugation. J Bras Reprod Assist. 2016;20:206–211. doi: 10.5935/1518-0557.20160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Soto JC, Landeras J, Gadea J. Spermatozoa and seminal plasma fatty acids as predictors of cryopreservation success. Andrology. 2013;1:365–375. doi: 10.1111/j.2047-2927.2012.00040.x. [DOI] [PubMed] [Google Scholar]
- 17.Patel M, Gandotra VK, Cheema RS, Bansal AK, Kumar A. Seminal plasma heparin binding proteins improve semen quality by reducing oxidative stress during cryopreservation of cattle bull semen. Asian-Australasian J Anim Sci. 2016;29:1247–1255. doi: 10.5713/ajas.15.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrios B, Pérez-Pé R, Muiño-Blanco T, Cebrián-Pérez JA. Seminal plasma proteins revert the cold-shock damage on ram sperm membrane. Int J Androl. 2001;24:352–359. doi: 10.1046/j.1365-2605.2001.00315.x. [DOI] [PubMed] [Google Scholar]
- 19.Hotaling J, Patel D, Vendryes C. Predictors of sperm recovery after cryopreservation in testicular cancer. Asian J. 2016:35–8. [DOI] [PMC free article] [PubMed]
- 20.Hamilton JAM, Cissen M, Brandes M, JMJ S, De Bruin JP, Kremer JAM, et al. Total motile sperm count: a better indicator for the severity of male factor infertility than the WHO sperm classification system. Hum Reprod. 2014;30:1110–1121. doi: 10.1093/humrep/dev058. [DOI] [PubMed] [Google Scholar]
- 21.Borges E, Setti AS, Braga DPAF, RCS F, Iaconelli A. Total motile sperm count has a superior predictive value over the WHO 2010 cut-off values for the outcomes of intracytoplasmic sperm injection cycles. Andrology. 2016;4:880–886. doi: 10.1111/andr.12199. [DOI] [PubMed] [Google Scholar]
- 22.Montagut M, Gatimel N, Bourdet-Loub??re S, Daudin M, Bujan L, Mieusset R, et al. Sperm freezing to address the risk of azoospermia on the day of ICSI. Hum Reprod 2015;30:2486–2492. [DOI] [PubMed]
- 23.Caponecchia L, Cimino G, Sacchetto R, Fiori C, Sebastianelli A, Salacone P, et al. Do malignant diseases affect semen quality? Sperm parameters of men with cancers. Andrologia Wiley Online Library. 2016;48:333–340. doi: 10.1111/and.12451. [DOI] [PubMed] [Google Scholar]
- 24.Auger J, Sermondade N, Eustache F. Semen quality of 4480 young cancer and systemic disease patients: baseline data and clinical considerations. Basic Clin Androl. 2016;26:3. doi: 10.1186/s12610-016-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daudin M, Rives N, Walschaerts M, Drouineaud V, Szerman E, Koscinski I, et al. Sperm cryopreservation in adolescents and young adults with cancer: results of the French national sperm banking network (CECOS) Fertil Steril. 2015;103:478–486.e1. doi: 10.1016/j.fertnstert.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal A, Allamaneni SSR. Disruption of spermatogenesis by the cancer disease process. J Natl Cancer Inst Monogr. 2005;44195:9–12. doi: 10.1093/jncimonographs/lgi005. [DOI] [PubMed] [Google Scholar]
- 27.Martini AC, Molina RI, Estofán D, Tissera A, Ruiz RD, de Cuneo MF. Improving the predictive value of the hypoosmotic swelling test in humans. Fertil Steril. 2016;85:1840–1842. doi: 10.1016/j.fertnstert.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 28.Nallella KP, Sharma RK, Allamaneni SSR, Aziz N, Agarwal A. Cryopreservation of human spermatozoa: comparison of two cryopreservation methods and three cryoprotectants. Fertil Steril. 2004;82:913–918. doi: 10.1016/j.fertnstert.2004.02.126. [DOI] [PubMed] [Google Scholar]
- 29.Donnelly ET, McClure N, Lewis SEM. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril. 2001;76:892–900. doi: 10.1016/S0015-0282(01)02834-5. [DOI] [PubMed] [Google Scholar]
- 30.Petyim S, Neungton C, Thanaboonyawat I, Laokirkkiat P, Choavaratana R. Sperm preparation before freezing improves sperm motility and reduces apoptosis in post-freezing-thawing sperm compared with post-thawing sperm preparation. J Assist Reprod Genet. 2014;31:1673–1680. doi: 10.1007/s10815-014-0332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esteves SC, Sharma RK, Thomas AJ, Agarwal A. Improvement in motion characteristics and acrosome status in cryopreserved human spermatozoa by swim-up processing before freezing. Hum Reprod. 2000;15:2173–2179. doi: 10.1093/humrep/15.10.2173. [DOI] [PubMed] [Google Scholar]
- 32.Brugnon F, Ouchchane L, Pons-Rejraji H, Artonne C, Farigoule M, Janny L. Density gradient centrifugation prior to cryopreservation and hypotaurine supplementation improve post-thaw quality of sperm from infertile men with oligoasthenoteratozoospermia. Hum Reprod. 2013;28:2045–2057. doi: 10.1093/humrep/det253. [DOI] [PubMed] [Google Scholar]