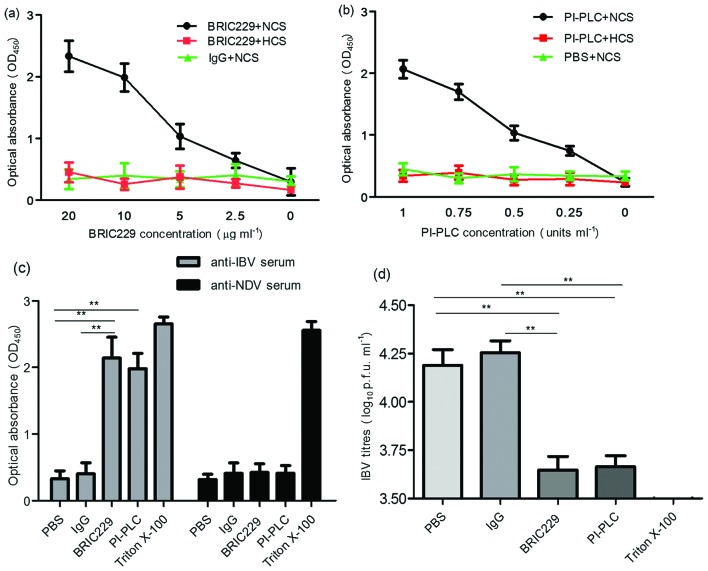
Fig. 3.
Sensitivity of IBV to ADCML. (a and b) Cell-free supernatant from IBV-infected H1299 cells was preincubated with mouse IgG, BRIC229 or PI-PLC at different concentrations, followed by the addition of anti-IBV pAbs plus normal chicken serum (NCS) or heat-inactivated chicken serum (HCS). IBV lysis was quantified by detecting the release of IBV N proteins using antibody sandwich enzyme-linked immunosorbent assay. Dose-dependent analysis of the IBV N proteins released from lysed IBV virions was performed in response to BRIC229 and PI-PLC treatments with anti-IBV pAbs in the presence or absence of the complement components. (c) The effects of antibody specificity on complement-mediated IBV lysis in response to treatment with PBS, mouse IgG, BRIC229, PI-PLC or triton X-100. Chicken-derived complement components in NCS were added in all groups with either anti-IBV pAbs or anti-NDV pAbs. (d) Reduction test for IBV infectivity. A reduction test for IBV infectivity was conducted by inoculating 100 % confluent H1299 cells with viral samples from the groups treated with anti-IBV pAbs in Fig. 3(c). The number of infectious IBV particles retained in the samples of the ADCML experiments was quantified by plaque assay.