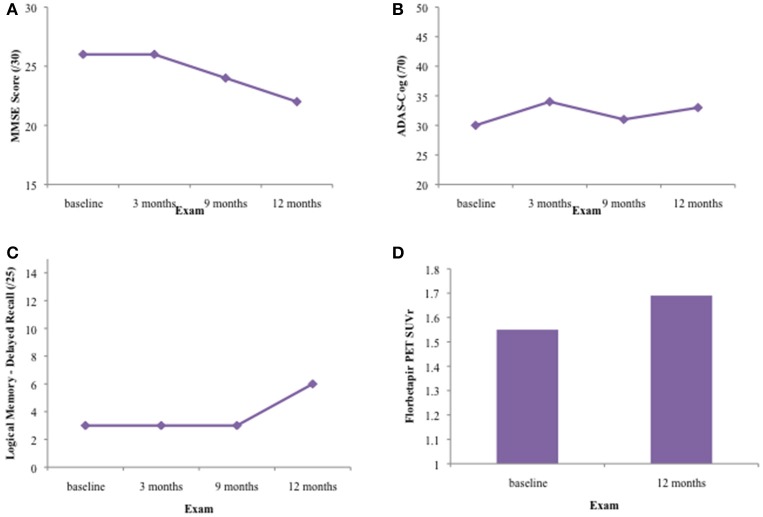
Figure 1.
Results on primary outcome measures for patient CS over 12 months of DFMO therapy. (A) Results on the Mini-Mental Status Exam (MMSE)/30 at baseline, 3, 6, and 12 month exams. CS' MMSE score declined 4 points over the 12 month treatment period, from 26/30 to 22/30, indicating generalized cognitive decline. (B) Results on the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) 11—item at baseline, 3, 6, and 12 month exams. Patient CS' ADAS-Cog score increased by 3 points over the 12 month treatment period, from 30/70 to 33/70. Higher scores on this test indicate increased cognitive impairment. (C) Results on the delayed recall portion of the Logical Memory stories at baseline, 3, 6, and 12 month exams. The patient showed impaired verbal learning and episodic memory throughout the 12 month treatment period. Although the patient recalled 6/25 items at the 12 month visit, this was not concurrent with results on other episodic memory tests such as the ADAS-Cog word list learning and recall (0/10 words at delayed recall) and the ISLT (2/12 shopping items at delayed recall). (D) SUVr on Florbetapir PET scan at baseline (1.55) and after 12 month treatment period (1.69).