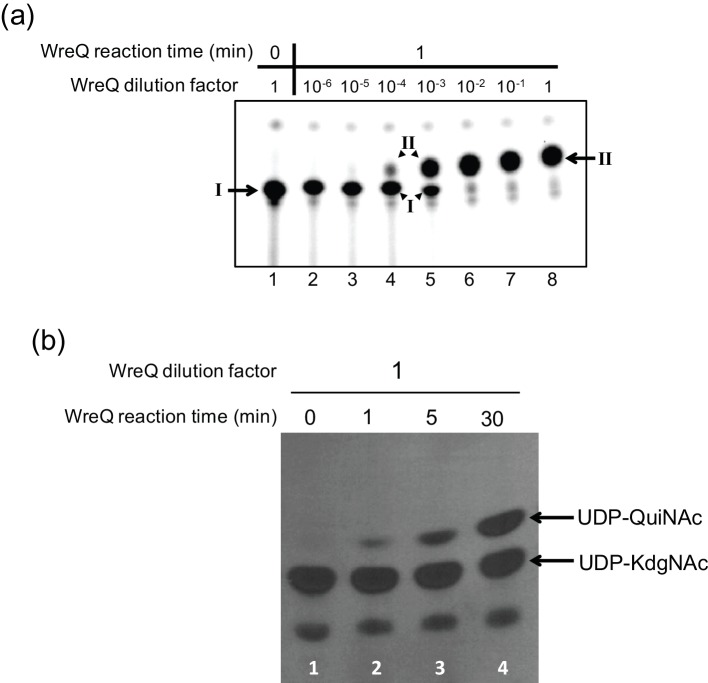
Fig. 4.
Comparison of the WreQ kinetic activity with lipid-linked versus nucleotide-linked substrates. Autoradiograms show products separated on TLCs after controlled times of incubation and concentrations of WreQ. (a) WreQ catalysis with the lipidated substrate. First, [32P] WreU transferase reactions with the UDP-KdgNAc substrate were allowed to proceed for 1 h (i.e. under the same conditions as for Fig. 2, lane 4). Then, WreQ and NADH were added. After 1 min, the reactions were terminated by adding and rapidly mixing with chloroform-methnol/3 : 2 (solvent I). Lanes: lane 1 is a 0 min control in which solvent I was added before WreQ; lanes 2–8 are groups that contain serially diluted WreQ, from 106 to 1 (undiluted). Inferred compounds: I, Und-PP-KdgNAc; II, Und-PP-QuiNAc. (b) WreQ catalysis with UDP-KdgNAc. WreQ and NADH were added to reactions in which UDP-[3H]KdgNAc was produced by complete conversion from UDP-[3H]GlcNAc catalysed by WbpM in a 30 min reaction [19]. WreQ concentration was the same as the undiluted concentration used in panel (a) (10 µg His6-WreQ per 100 µl reaction). Catalysis was allowed to proceed for different times before being terminated by boiling. Lanes: lane 1, 0 min; lane 2, 1 min; lane 3, 5 min; lane 4, 30 min.