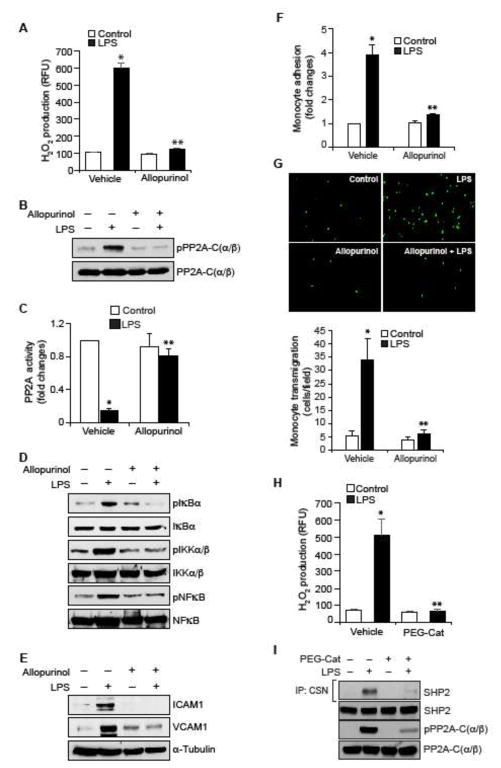
Figure 5. RvD1 inhibits LPS-induced XO-mediated ROS production to prevent PP2A inactivation, NFκB activation, ICAM1 and VCAM1 expression and EC-monocyte interactions.
A. Quiescent HUVECs were treated with and without LPS (500 ng/ml) in the presence and absence of Allopurinol (50 μM), a specific inhibitor of XO, for 10 min and assayed for H2O2 production. B, D & E. Quiescent HUVECs were treated with and without LPS (500 ng/ml) in the presence and absence of Allopurinol (50 μM) for 30 min or 1 hr and equal amounts of protein from each condition of 30 min treatment samples were analyzed by Western blotting for pPP2A-C(α/β), pIκBα, pIKKα/β and pNFκB levels and 1 hr treatment samples were analyzed for ICAM1 and VCAM1 levels and the blots were normalized for their total levels or α-tubulin. C. The cell extracts of panel B were assayed for PP2A activity. F & G. Quiescent HUVEC monolayer was treated with and without LPS (500 ng/ml) in the presence and absence of Allopurinol (50 μM) for 2 hrs and assayed for THP1 cell adhesion (F) or transmigration (G) as described in Figure 1E and F, respectively. H & I. Quiescent HUVEC monolayer was treated with and without LPS (500 ng/ml) in the presence and absence of PEG-catalase (50 U/ml) for 10 min for H2O2 production or 30 min for cell extracts preparation. To measure SHP2 cysteine oxidation, cell extracts containing equal amounts of protein from each condition were immunoprecipitated with cysteine sulfonate (CSN) antibodies and the immunocomplexes were immunoblotted for SHP2 using its specific antibodies. The same cell extracts were also analyzed by Western blotting for total SHP2. PP2A-C(α/β) Tyr phosphorylation was measured by Western blotting using its phospho-specific antibodies and the blot was normalized to its total levels. *, p < 0.05 vs control; **, p < 0.05 vs LPS. PEG-Cat, PEG-catalase.