Figure 7.
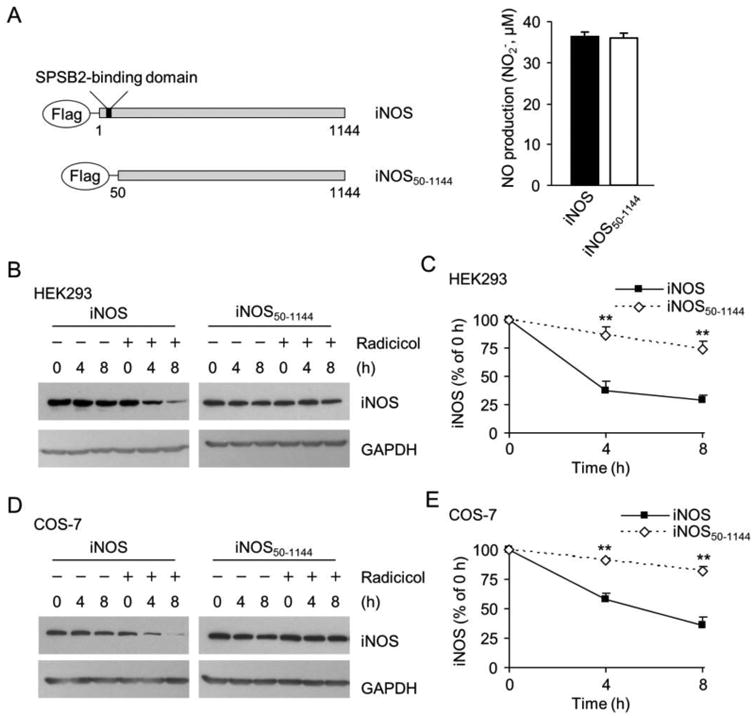
Deletion of SPSB2-interacting domain on iNOS prevents iNOS degradation in Hsp90-inhibited cells. (A) A schematic representation of wild-type iNOS and SPSB2-binding domain deficient mutant (iNOS50-1144). Wild-type and iNOS50-1144 exhibited the same NO-generating activity. Data are mean ± SE, n = 4. (B) Hsp90 inhibition-induced iNOS degradation in HEK 293 cells was significantly decelerated by deleting the SPSB2-binding domain on iNOS. (C) Quantitative analyses of the effects of deleting SPSB2-interacting domain from iNOS on its degradation in Hsp90-inhibited HEK293 cells. **P < 0.01 vs. control, n = 3. (D) Deletion of the SPSB2-binding domain also prevented the degradation of iNOS in COS-7 cells after Hsp90 inhibition (Radicicol, 20 μM). (E) Quantitative analyses of the turnover rate of wild-type and iNOS50-1144 in Hsp90-inhibited COS-7 cells. **P < 0.01 vs. control, n = 3.
