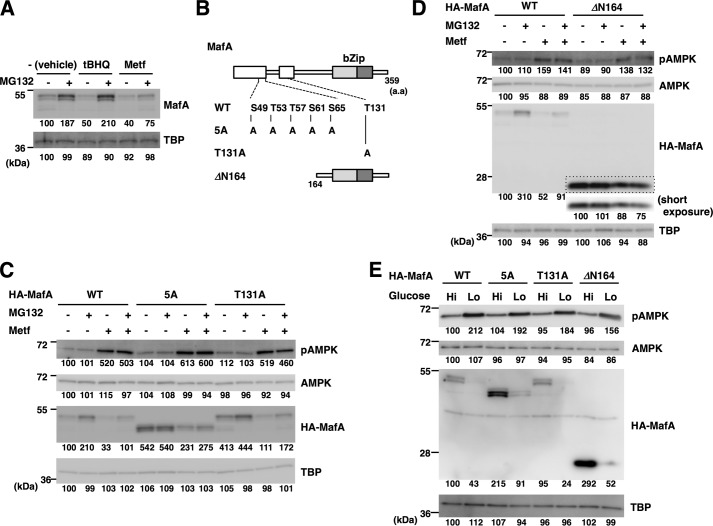
Figure 6.
AMPK promotes MafA protein degradation independent of the proteasome and multiple phosphorylation events. A, oxidative stress, but not AMPK, promotes proteasomal MafA degradation. MIN6 cells were treated with metformin (Metf, 500 μm) or the inducer of oxidative stress tBHQ (5 μm) with or without the proteasome inhibitor MG132 (5 μm) for 16 h. Cell extracts were then subjected to immunoblot analysis. B, schematics of MafA mutants. The boxed areas represent the evolutionarily conserved domains. bZip, basic leucine zipper. C and D, WT or mutant forms of HA-tagged MafA (5A, T131A, and ΔN164) shown in B were expressed in MIN6 cells. The electrophoretic mobility of the 5A mutant is higher than WT MafA because of loss of phosphorylation events at five Ser/Thr residues. Cells were then treated with or without metformin (500 μm) and MG132 (5 μm) for 16 h and were analyzed by immunoblotting. Short exposure of the boxed area of the anti-HA blot (D) was also indicated. E, WT or mutant forms of HA-tagged MafA (5A, T131A, and ΔN164) were expressed in MIN6 cells. Cells were then grown in high-glucose (Hi, 20 mm) or low-glucose (Lo, 2 mm) medium for 16 h and analyzed by immunoblot analysis. The data in A and C–E are representative of two independent biological experiments. The intensity of the bands was quantified using ImageJ software, and the relative amounts (averages of two independent experiments) are indicated below the bands.