Figure 4.
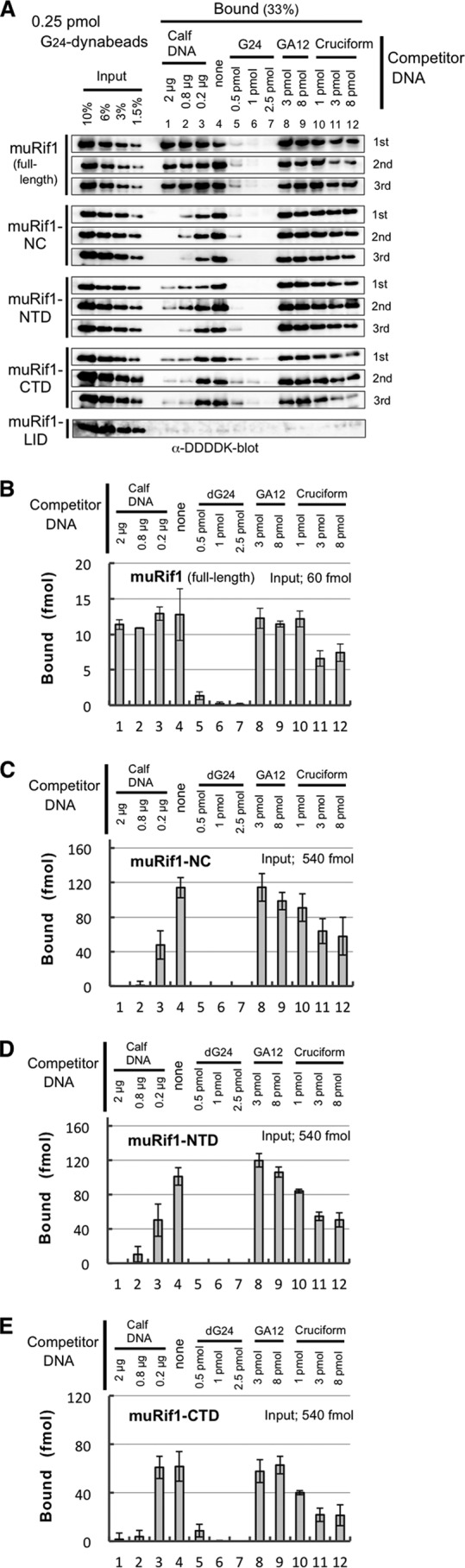
Specific association of muRif1 domains with immobilized G4 DNA. A 5′-biotinylated, G4-folded T6G24 oligonucleotide was conjugated to streptavidin-dynabeads (G24-dynabeads) and used to pull down partially-purified muRif1 or truncated muRif1 polypeptides (Fig. S3). The same experiment was repeated three times for every polypeptide (A) except muRif1-LID. After densitometric scanning of the immunoblots, the amounts of G4 DNA-bound muRif1 (B), muRif1-NC (C), muRif1-NTD (D), and muRif1-CTD (E) are plotted in the bar graphs. A cruciform competitor was made of four oligonucleotides of 40 nucleotides long, identical to those used in Sukackaite et al. (26). The presence of a large excess of T6(GA)12 DNA (3–8 pmol; lanes 8 and 9) or 1 pmol of the cruciform DNA (lane 10) did not significantly affect the bindings of these muRif1 polypeptides to the beads (B–E), whereas addition of >0.5 pmol of G4-folded T6G24 DNA competed out the bindings (lanes 5–7). Addition of >3 pmol of the cruciform DNA reduced the bindings (lane 11 and 12), but less efficiently than the G4 competitor did.
