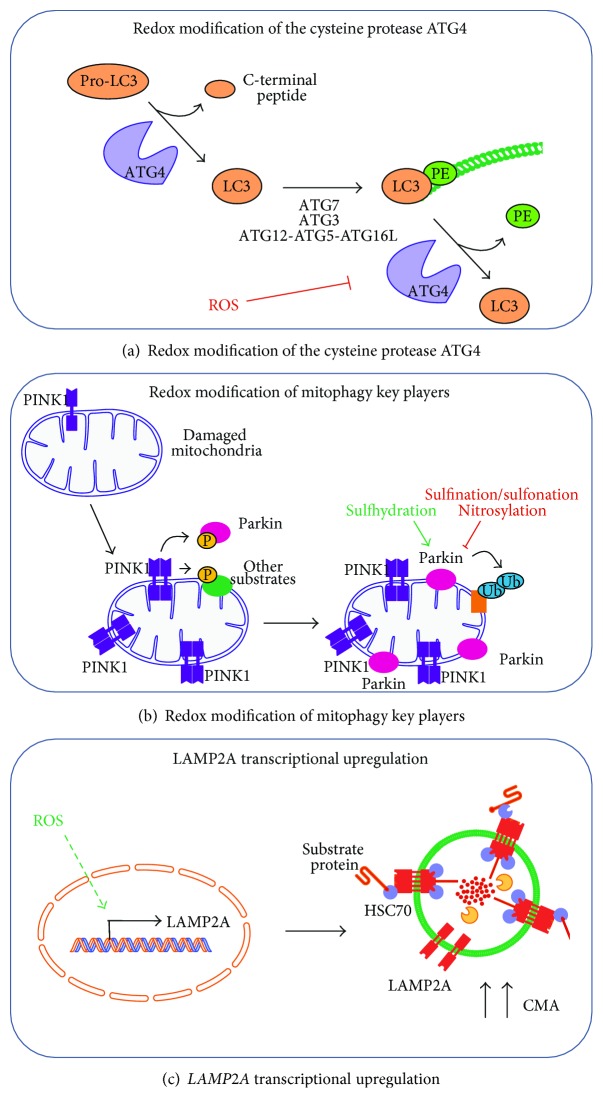
Figure 1.
Redox modification of autophagy core components. (a) Cysteine protease ATG4 is sensitive to redox modification. ATG4 cleaves the C-terminal peptide in LC3 (or GABARAPs), making it a suitable substrate for conjugation to phosphatidylethanolamine (PE), which is mediated by ATG7, ATG3, and the ATG12-ATG5-ATG16L complex. LC3 conjugated to PE (LC3-II) is inserted into the autophagosomal membrane and enables it to elongate. ATG4 also acts as a delipidating enzyme, releasing LC3 from PE. ROS are essential for regulating ATG4 activity, as redox modification of cysteine residues transiently inhibits delipidation activity in order to promote autophagosome formation. (b) Mitophagy core components are targets of redox modification. Briefly, damaged mitochondria result in the stabilisation, dimerisation, and activation of kinase PINK1 in the organelle. PINK1 phosphorylates Parkin and other substrates, which further recruit Parkin to the mitochondrial membrane. Parkin acts as an E3-ubiquitin ligase, ubiquitinating several substrates that are recognised by autophagy receptors in order to direct mitochondria toward lysosomal degradation. Physiological sulfhydration enables, whereas pathological nitrosylation or sulphination/sulfonation inhibits, Parkin catalytic activity. (c) Mild oxidative stress upregulates chaperone-mediated autophagy (CMA) by transcriptional induction of lysosomal receptor LAMP2A.