Abstract
Purpose of Review
The purpose of this review is to define pulmonary hypertension in the setting of left heart disease (PH-LHD), discuss its epidemiology and pathophysiology, and highlight the cause and effect relationship it has with disease progression in the setting of cardiomyopathy.
Recent Findings
Both pulmonary hypertension (PH) and heart failure are becoming increasingly common. As such, PH-LHD is now the most common form of PH. The pathophysiology of the condition relates to backward transmission of elevated left ventricular filling pressures into the pulmonary circulation and, ultimately, right ventricular (RV) strain/dysfunction. It is evident that these pathophysiologic processes are both the effect and cause of left heart disease progression.
Summary
In this review, we describe the complex relationship between disease progression in left ventricular cardiomyopathy and PH-LHD. Clinicians and researchers should take note of the importance of PH-LHD and RV dysfunction to appropriately risk stratify patients and develop therapies for the condition.
Keywords: Pulmonary hypertension, Left heart disease, Heart failure, WHO group 2, Cardiomyopathy, Valvular heart disease
Introduction
Heart failure (HF) is a progressive clinical syndrome which is associated with significant morbidity and mortality, yielding a substantial economic burden [1]. An estimated 5.7 million Americans currently carry the diagnosis of HF with an anticipated 46% rise in prevalence by 2030 [2]. Furthermore, the cost of caring for these patients is enormous and currently estimated to be $30.7 billion/year with projections indicating that amount will rise to $69.7 billion/year by 2030 [2]. As per the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline for the Management of Heart Failure, HF can be classified as either HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF, previously referred to as diastolic HF) [3]. Those with HFrEF have a left ventricular ejection fraction (LVEF) ≤ 40% while those with HFpEF have an LVEF ≥ 50% [3]. Both patients with HFrEF (more specifically, those with a left ventricular cardiomyopathy) as well as HFpEF can and do develop pulmonary hypertension (PH-LHD), although this review will focus on those with HFrEF and pulmonary hypertension (PH), and specifically the interaction between the two. Prior to addressing the interaction between these two disorders, however, it would be useful to further describe the entity of PH.
Pulmonary Hypertension: a Brief Overview
PH is a heterogeneous condition comprised of multiple and wide-ranging etiologies characterized by a mean pulmonary artery pressure (mPAP) ≥ 25 mmHg as assessed by resting right heart catheterization [4••]. There are several complementary methods with which to define PH including (1) clinical classifications (otherwise known as World Health Organization [WHO] groups) and (2) hemodynamic classifications (Table 1). Pulmonary arterial hypertension (PAH; WHO group I), specifically, is a rare, progressive disorder which results in a primary pulmonary vasculopathy in the absence of overt left ventricular diastolic, systolic, or valvular dysfunction, and culminates in right ventricular (RV) failure and death. While treatment for PAH has improved drastically over the last two decades, the same cannot be said for other forms of PH, especially PH-LHD.
Table 1.
Hemodynamic classifications of pulmonary hypertension [4]
| Definition | Characteristicsa | Clinical groups |
|---|---|---|
| PH | PAPm ≥ 25 mmHg | All |
| Pre-capillary PH | PAPm ≥ 25 mmHg | 1. Pulmonary arterial hypertension |
| PAWP ≤ 15 mmHg | 3. PH due to lung diseases | |
| 4. Chronic thromboembolic PH | ||
| 5. PH with unclear and/or multifactorial mechanisms | ||
| Post-capillary PH | PAPm ≥ 25 mmHg | 2. PH due to left heart disease |
| Isolated post-capillary PH (Ipc-PH) | PAWP > 15 mmHg | 5. PH with unclear and/or multifactorial mechanisms |
| Combined post-capillary and pre-capillary PH (Cpc-PH) | DPG < 7 mmHg and/or PVR ≤ 3 WU | |
| DPG ≥ 7 mmHg and/or PVR 3 WU |
All values measured at rest. DPG diastolic pressure gradient (diastolic PAP—mean PAWP), PAPm mean pulmonary artery pressure, PAWP pulmonary artery wedge pressure, PH pulmonary hypertension, PVR pulmonary vascular resistance, WU Wood units
Pulmonary Hypertension Due to Left Heart Disease
Hemodynamic Landscape
PH-LHD, the most common type of PH, is defined as an mPAP of ≥ 25 mmHg and a pulmonary artery wedge pressure (PAWP) > 15 mmHg in the setting of a normal or reduced cardiac output [5]. Furthermore, PH-LHD can be the result of isolated pulmonary venous hypertension or “passive” PAWP elevation, which has recently been classified as isolated post-capillary PH (Ipc-PH) if the diastolic pressure gradient (DPG; diastolic pulmonary artery pressure—PAWP) is < 7 mmHg and/or pulmonary vascular resistance (PVR) is ≤ 3 Wood units (WU). PH-LHD is considered to be combined pre- and post-capillary (Cpc-PH) in nature when the DPG ≥ 7 mmHg and/or the PVR is > 3 WU [6••]. Cpc-PH has traditionally been referred to as “reactive”, “mixed”, or “out-of-proportion” PH and is often thought to represent excess vasoconstriction with or without vascular remodeling, all leading to a “disproportionate” increase in pulmonary artery pressure (PAP) [6••]. The DPG has been proposed as a metric to help prognosticate survival in those with Cpc-PH and was shown by Gerges et al. [7] to predict worse median survival when ≥ 7 mmHg, an effect that has not borne out universally across other studies [8–10]. Prognostic assertions aside, several studies in a variety of populations have shown that Cpc-PH may share more features with PAH including mortality and histopathology as well as genetic and pathophysiologic mechanisms as compared to Ipc-PH [7, 11–14]. Furthermore, echo-Doppler parameters including TAPSE, right ventricular outflow tract pulse-wave Doppler notching, acceleration time, presence of systolic septal flattening, among other metrics of RV (relative to LV) structure and function, as well as cardiopulmonary exercise testing parameters can be informative in delineating the phenotype of PH-LHD that is present (Fig. 1) [14–16].
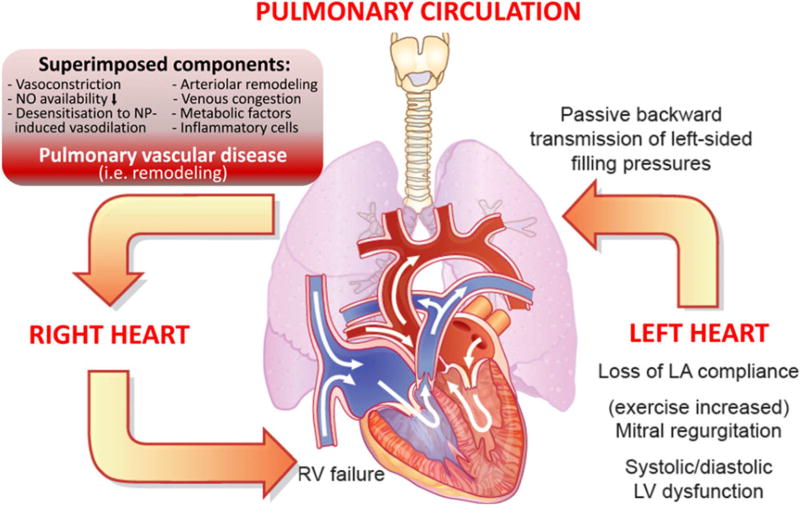
Fig. 1.
Cardiopulmonary interaction and pathobiology of pulmonary hypertension (PH) in left ventricular heart failure. Shown is (i) the backward transmission of elevated left ventricular filling pressures into the pulmonary circulation (post-capillary hemodynamic profile), (ii) potential superimposed components contributing to the extent of PH (leading to a pre-capillary component), which may be associated with (iii) pulmonary vascular remodeling in some patients, thus leading to (iv) right ventricular strain and dysfunction over time. Right ventricular (RV) dilation and increase in wall stress/tension (internal RV afterload) result in elevated myocardial oxygen consumption, which with concomitant reduction in coronary perfusion gradient leads to RV ischemia and progressive RV failure [6]
Epidemiology
As a result of this broad range of heterogeneous definitions, it is difficult to decipher the true global epidemiology of PH; however, PH-LHD appears to now be the most common form of the condition [5], accounting for 65–80% of cases [6••]. Furthermore, study populations vary, as do the methods of diagnosing PH in a given study (i.e., echo-Doppler diagnosis vs. right heart catheterization). As noted above, PH-LHD can manifest across the HF spectrum, from HFpEF to HFrEF, and across AHA/ACC disease stages [3]. In HfpEF, which accounts for approximately 50% of all patients with HF [17], the prevalence of PH-LHD ranges widely from 36 to 83% as assessed by both echocardiography and right heart catheterization [18–20]. In one representative study, Lam et al. demonstrated that an elevated echocardiographic pulmonary artery systolic pressure (PASP; > 35 mmHg) was detected in 83% of a cohort of 224 HFpEF patients [18]. When present, PH-LHD could be potentiated by many co-morbidities including diabetes mellitus, obstructive sleep apnea, obesity (potentially mediated by decreased natriuretic peptide sensitivity), genetics, and gender, among others [21]. This “second-hit” type hypothesis is potentially relevant in explaining why PH-LHD is not present in every patient with HFpEF.
PH-LHD has also been noted to be particularly prevalent in the setting of advanced HFrEF as well as left-sided valvular heart disease (i.e., mitral valve disease) which can often lead to a Cpc-PH phenotype [11, 22]. The presence of PH-LHD in the former group can be particularly important and challenging in the context of consideration of mechanical circulatory support or orthotopic heart transplantation. Specifically regarding PH in HFrEF, epidemiological data are available but have been limited mainly to those with AHA stage D (advanced) HF [23]. In a cohort of 320 patients, Butler et al. found that 28% had a normal PVR (pulmonary vascular resistance) (< 1.5 Wood units), 36% had a mildly elevated PVR (1.5–2.49 Wood units), 17% had a moderately elevated PVR (2.5–3.49 Wood units), and 19% had a severely elevated PVR (> 3.5 Wood units) [24]. In their cohort of patients with unexplained cardiomyopathy, Tampakakis et al. found that 469 of 1174 patients (40%) had a mean pulmonary artery pressure ≥ 25 mmHg consistent with PH-LHD [8].
Pathophysiology
In all of these settings, the pathophysiology of PH-LHD is thought to be similar: backward transmission of elevated left ventricular filling pressures into the pulmonary circulation followed by potential superimposed components (such as pulmonary vasoconstriction, decreased nitric oxide availability, and desensitization to natriuretic peptide-induced vasodilatation) contributing to the extent of PH. This leads to pulmonary vascular remodeling and, ultimately, right ventricular (RV) strain/dysfunction (Fig. 2) [6••]. RV strain results in increased wall stress/tension as well as elevated myocardial oxygen consumption, all culminating in RV ischemia and failure [6••]. Of note, elevated pulmonary pressures in PH-LHD patients as estimated by Doppler echocardiography have been shown by Bursi et al. to be a strong and independent predictor of all-cause as well as CV death in dose-response fashion [25]. Thus, the presence of PH in the setting of HF, regardless of etiology, predicts increased morbidity and mortality [23, 26]. The remainder of this paper will explore the complex cause and effect relationship between PH-LHD and disease progression in left ventricular cardiomyopathy.
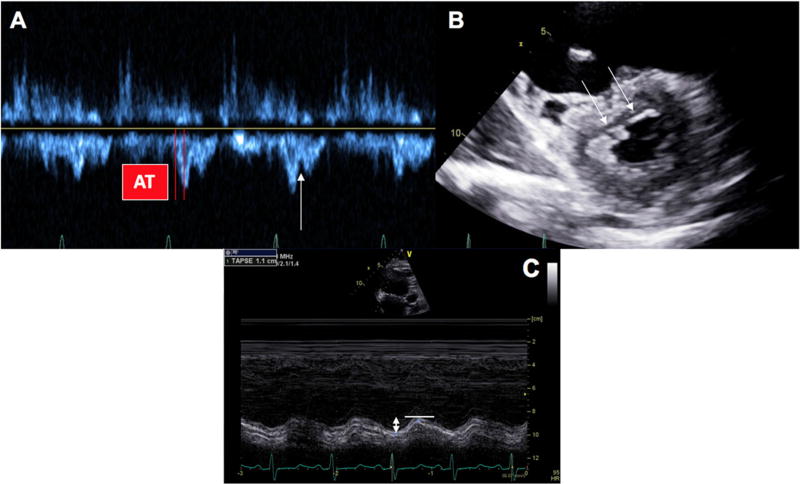
Fig. 2.
Echocardiographic manifestations of Cpc-PH. a reveals a pulmonary valve acceleration time (AT) as well as a right ventricular outflow tract pulse-wave spectral Doppler notch (arrow). b reveals systolic interventricular septal flattening (double arrows). Finally, c illustrates a low tricuspid annular systolic plane excursion (double-headed arrow) which is indicative of decreased right ventricular function. All three of these features are consistent with the presence of Cpc-PH rather than Ipc-PH
Is Pulmonary Hypertension the Result or Cause of Disease Progression in Left Ventricular Cardiomyopathy?
Result
PH-LHD specifically in the setting of cardiomyopathy and HFrEF exhibits a prevalence of 40–75% as assessed by right heart catheterization [8, 24, 27, 28]. Although a cause/effect relationship is difficult to establish, is the PH in this setting an associated consequence of a worsening cardiomyopathy, or, rather, is it causally implicated in disease progression? At least initially, PH-LHD is likely a result of worsening left-sided cardiomyopathy. As left-sided filling pressures increase (measured by PAWP and left ventricular end-diastolic pressure) or as the severity of mitral regurgitation increases, the severity of PH increases linearly [29, 30]. Time is also a factor in this process—the longer the pulmonary circulation is exposed to high left-sided filling pressures, the more predisposed vessels will be to developing structural and pathophysiological changes. Of note, if the left ventricle (LV) is unloaded and filling pressures are decreased (by LV assist device therapy, for example), pulmonary artery pressures and PVR may decline or even normalize [31, 32]. If not, additional structural changes that may occur include thickening of the alveolar-capillary membrane, medial hypertrophy, intimal and adventitial fibrosis, and luminal occlusion in small vessels [6••, 33, 34]. Interestingly, many of these changes may actually be adaptive and potentially protective against pulmonary edema in the setting of chronically elevated left ventricular filling pressures [34]. As demonstrated by Tedford et al. in this context, pulmonary vascular resistance increases, pulmonary artery compliance decreases, and right ventricular pulsatile load increases [35]. These increases in both RV pulsatile and resistive load induce structural and functional changes in the RV, as described below [6••]. From this point on, however, it could be argued that PH-LHD, and specifically with its impact on RV function, begins to contribute to LV cardiomyopathy disease progression.
Cause
As the severity of left ventricular dysfunction progresses, the RV begins to uncouple from the pulmonary artery, leading to right ventricular dysfunction [6••]. RV dilatation in this setting results in increased wall stress, enhanced myocardial oxygen demand, and provoked ischemia as well as symptoms of effort angina [23, 36]. The overall phenotype of the cardiomyopathy then begins to change, first from isolated left ventricular dysfunction to biventricular dysfunction, and then potentially to a right ventricular failure-predominant phenotype [6••]. This RV failure phenotype is complicated by systemic venous congestion, renal dysfunction, and ascites [23].
There is strong evidence that the development of right-sided dysfunction heralds a new stage of disease progression associated with a worse functional capacity as well as overall prognosis. Guazzi et al. demonstrated that those patients with heart failure and low tricuspid annular plane systolic excursion (TAPSE; indicative of decreased right ventricular function) paired with a high PASP, resulting in a reduced TAPSE/PASP ratio as a marker of RV-PA uncoupling [37], had a 5.6 times higher hazard of major cardiac events as compared to those patients who did not exhibit those characteristics [38]. Additionally, that high-risk group of patients performed poorly on CPET with the lowest peak VO2 as well as highest end-oscillatory ventilation rate [38]. Butler et al. also demonstrated a statistically significantly lower peak exercise VO2 associated with increasing PVR in 320 patients with HFrEF [24], indicating poor functional and prognostic status. Moreover, an increased minute ventilation (VE)/carbon dioxide production (VCO2) relationship, a marker of ventilatory inefficiency, is a CPET parameter suggestive of both pulmonary vascular disease as well as significant RV dysfunction in the setting of LV cardiomyopathy [39, 40]. This parameter has been shown to be prognostically additive to peak VO2 in patients with end-stage HFrEF and can predict survival or time to transplant. Finally, Bosch and colleagues utilized the RV longitudinal strain (as a measure of right ventricular function) to PASP ratio alongside the TAPSE/PASP ratio to demonstrate both measures, after multivariate adjustment, were statistically significantly associated with a higher hazard of all-cause mortality and heart failure hospitalization for those with HFrEF vs. controls, independent of PASP [41].
Thus, it is important to note, that while a progression from an LV cardiomyopathy-predominant phenotype to a biventricular or RV dysfunction-predominant phenotype is often in the setting of PH-LHD, there are other factors associated with this progression, including changes in septal dynamics (reverse Bernheim effect) [42] or ventricular interdependence [23]. In fact, Ghio et al. recently again showed the prognostic importance of TAPSE/PASP ratio across the HF spectrum (HFrEF and HFpEF), yet there were different correlates of RV dysfunction in HFrEF compared to HFmrEF and HFpEF [43]. Specifically, correlates of RV dysfunction (reduced TAPSE) in HFrEF included non-sinus rhythm, high heart rate, ischemic etiology, and E-wave deceleration time < 140 ms, whereas PASP > 40 mmHg was the strongest correlate of a reduced TAPSE in HFpEF. This suggests that RV dysfunction, specifically in HFrEF, may be independent of PH-LHD, though the presence of RV dysfunction and resultant RV-PA uncoupling still portends a worse prognosis than isolated LV dysfunction. Lastly, Dini et al. recently demonstrated that reversal of RV dysfunction as illustrated by an improved TAPSE was associated with statistically significantly improved survival, also pointing to an association of RV dysfunction with overall disease progression [44].
Conclusion
As the prevalence of both HFrEF and HFpEF rises over time, the phenomenon of PH-LHD will continue to become more common in clinical practice. It is clear that not only is PH-LHD caused by worsening left-sided heart disease but that it is an integral part of the progression, both functionally and prognostically, of the clinical syndrome of HF. Clinicians should take note of the importance of PH-LHD and resultant/coexistent RV dysfunction to appropriately risk stratify patients, allowing for optimal treatment approaches and outcomes in this population.
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest The authors declare that they have no conflicts of interest.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.Mazurek JA, Jessup M. Understanding heart failure. Heart Fail Clin. 2017;13:1–19. doi: 10.1016/j.hfc.2016.07.001. https://doi.org/10.1016/j.hfc.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2016 update. Circulation. 2015 doi: 10.1161/CIR.0000000000000350. CIR.0000000000000350; https://doi.org/10.1161/CIR.0000000000000350. [DOI] [PubMed]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. https://doi.org/10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4••.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. https://doi.org/10.1093/eurheartj/ehv317. This is an excellent recent guideline document on the topic of pulmonary hypertension. [DOI] [PubMed] [Google Scholar]
- 5.Vachiéry J-L, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–8. doi: 10.1016/j.jacc.2013.10.033. https://doi.org/10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 6••.Rosenkranz S, Gibbs JSR, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiéry J-L. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37:942–54. doi: 10.1093/eurheartj/ehv512. https://doi.org/10.1093/eurheartj/ehv512. This is an excellent update article on the specific topic of PH-LHD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, et al. Diastolic pulmonary vascular pressure gradient. Chest. 2013;143:758–66. doi: 10.1378/chest.12-1653. https://doi.org/10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 8.Tampakakis E, Leary PJ, Selby VN, Marco TD, Cappola TP, Felker GM, et al. The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. JACC Heart Fail. 2015;3:9–16. doi: 10.1016/j.jchf.2014.07.010. https://doi.org/10.1016/j.jchf.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tedford RJ, Beaty CA, Mathai SC, Kolb TM, Damico R, Hassoun PM, et al. Prognostic value of the pre-transplant diastolic pulmonary artery pressure to pulmonary capillary wedge pressure gradient (DPG) in cardiac transplant recipients with pulmonary hypertension. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2014;33:289–97. doi: 10.1016/j.healun.2013.11.008. https://doi.org/10.1016/j.healun.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail. 2015;3:467–74. doi: 10.1016/j.jchf.2015.01.013. https://doi.org/10.1016/j.jchf.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerges M, Gerges C, Pistritto A-M, Lang MB, Trip P, Jakowitsch J, et al. Pulmonary hypertension in heart failure. epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med. 2015;192:1234–46. doi: 10.1164/rccm.201503-0529OC. https://doi.org/10.1164/rccm.201503-0529OC. [DOI] [PubMed] [Google Scholar]
- 12.Assad TR, Hemnes AR, Larkin EK, Glazer AM, Xu M, Wells QS, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol. 2016;68:2525–36. doi: 10.1016/j.jacc.2016.09.942. https://doi.org/10.1016/j.jacc.2016.09.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazurek JA, Horne BD, Saeed W, Sardar MR, Zolty R. Galectin-3 levels are elevated and predictive of mortality in pulmonary hypertension. Heart Lung Circ. 2017 doi: 10.1016/j.hlc.2016.12.012. https://doi.org/10.1016/j.hlc.2016.12.012. [DOI] [PubMed]
- 14.Caravita S, Faini A, Deboeck G, Bondue A, Naeije R, Parati G, et al. Pulmonary hypertension and ventilation during exercise: role of the pre-capillary component. J Heart Lung Transplant. 2017;36:754–62. doi: 10.1016/j.healun.2016.12.011. https://doi.org/10.1016/j.healun.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Mazurek JA, Forfia PR. Enhancing the accuracy of echocardiography in the diagnosis of pulmonary arterial hypertension: looking at the heart to learn about the lungs. Curr Opin Pulm Med. 2013;19:437–45. doi: 10.1097/MCP.0b013e3283645966. https://doi.org/10.1097/MCP.0b013e3283645966. [DOI] [PubMed] [Google Scholar]
- 16.Mazurek JA, Vaidya A, Grandin EW, Forfia P. RVOT Doppler notching predicts diastolic-to-wedge gradient in left heart disease-associated pulmonary hypertension. J Am Coll Cardiol. 2015;65:A1537. https://doi.org/10.1016/S0735-1097(15)61537-6. [Google Scholar]
- 17.Lam CSP, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–70. doi: 10.1161/CIRCULATIONAHA.108.838698. https://doi.org/10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–26. doi: 10.1016/j.jacc.2008.11.051. https://doi.org/10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung CC, Moondra V, Catherwood E, Andrus BW. Prevalence and risk factors of pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fraction. Am J Cardiol. 2010;106:284–6. doi: 10.1016/j.amjcard.2010.02.039. https://doi.org/10.1016/j.amjcard.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 20.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, et al. Cardiac structure and function in heart failure with preserved ejection FractionClinical Perspective. Circ Heart Fail. 2014;7:104–15. doi: 10.1161/CIRCHEARTFAILURE.113.000887. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farr G, Shah K, Markley R, Abbate A, Salloum FN, Grinnan D. Development of pulmonary hypertension in heart failure with preserved ejection fraction. Prog Cardiovasc Dis. 2016;59:52–8. doi: 10.1016/j.pcad.2016.06.002. https://doi.org/10.1016/j.pcad.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Zotter-Tufaro C, Duca F, Kammerlander AA, Koell B, Aschauer S, Dalos D, et al. Diastolic pressure gradient predicts outcome in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2015;66:1308–10. doi: 10.1016/j.jacc.2015.07.011. https://doi.org/10.1016/j.jacc.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–90. doi: 10.1161/CIRCULATIONAHA.111.085761. https://doi.org/10.1161/CIRCULATIONAHA.111.085761. [DOI] [PubMed] [Google Scholar]
- 24.Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999;34:1802–6. doi: 10.1016/s0735-1097(99)00408-8. https://doi.org/10.1016/S0735-1097(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 25.Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CSP, Weston SA, et al. Pulmonary pressures and death in heart failure. J Am Coll Cardiol. 2012;59:222–31. doi: 10.1016/j.jacc.2011.06.076. https://doi.org/10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salamon JN, Kelesidis I, Msaouel P, Mazurek JA, Mannem S, Adzic A, et al. Outcomes in World Health Organization group II pulmonary hypertension: mortality and readmission trends with systolic and preserved ejection fraction–induced pulmonary hypertension. J Card Fail. 2014;20:467–75. doi: 10.1016/j.cardfail.2014.05.003. https://doi.org/10.1016/j-cardfail.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction. JACC Heart Fail. 2013;1:290–9. doi: 10.1016/j.jchf.2013.05.001. https://doi.org/10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–8. doi: 10.1016/s0735-1097(00)01102-5. https://doi.org/10.1016/S0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 29.Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail. 2004;10:285–91. doi: 10.1016/j.cardfail.2003.12.006. https://doi.org/10.1016/j-cardfail.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Guazzi M, Labate V. Pulmonary hypertension in heart failure patients: pathophysiology and prognostic implications. Curr Heart Fail Rep. 2016;13:281–94. doi: 10.1007/s11897-016-0306-8. https://doi.org/10.1007/s11897-016-0306-8. [DOI] [PubMed] [Google Scholar]
- 31.Kutty RS, Parameshwar J, Lewis C, Catarino PA, Sudarshan CD, Jenkins DP, et al. Use of centrifugal left ventricular assist device as a bridge to candidacy in severe heart failure with secondary pulmonary hypertension. Eur J Cardiothorac Surg. 2013;43:1237–42. doi: 10.1093/ejcts/ezs678. https://doi.org/10.1093/ejcts/ezs678. [DOI] [PubMed] [Google Scholar]
- 32.Lundgren J, Algotsson L, Kornhall B, Rådegran G. Preoperative pulmonary hypertension and its impact on survival after heart transplantation. Scand Cardiovasc J. 2014;48:47–58. doi: 10.3109/14017431.2013.877153. https://doi.org/10.3109/14017431.2013.877153. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, et al. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: heart failure causes severe lung disease. Hypertension. 2012;59:1170–8. doi: 10.1161/HYPERTENSIONAHA.111.186072. https://doi.org/10.1161/HYPERTENSIONAHA.111.186072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delgado JF, Delgado JF. The pulmonary circulation in heart failure. Rev Esp Cardiol. 2010;63:334–45. doi: 10.1016/s1885-5857(10)70066-9. https://doi.org/10.1016/S1885-5857(10)70066-9. [DOI] [PubMed] [Google Scholar]
- 35.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–97. doi: 10.1161/CIRCULATIONAHA.111.051540. https://doi.org/10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle–pulmonary circulation unit. Circulation. 2009;120:992–1007. doi: 10.1161/CIRCULATIONAHA.106.674028. https://doi.org/10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- 37.Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol - Heart Circ Physiol. 2013;305:H1373–81. doi: 10.1152/ajpheart.00157.2013. https://doi.org/10.1152/ajpheart.00157.2013. [DOI] [PubMed] [Google Scholar]
- 38.Guazzi M, Naeije R, Arena R, Corrà U, Ghio S, Forfia P, et al. Echocardiography of right ventriculoarterial coupling combined with cardiopulmonary exercise testing to predict outcome in heart failure. Chest. 2015;148:226–34. doi: 10.1378/chest.14-2065. https://doi.org/10.1378/chest.14-2065. [DOI] [PubMed] [Google Scholar]
- 39.Methvin AB, Owens AT, Emmi AG, Allen M, Wiegers SE, Dries DL, et al. Ventilatory inefficiency reflects right ventricular dysfunction in systolic heart failure. Chest. 2011;139:617–25. doi: 10.1378/chest.10-0318. https://doi-org/10.1378/chest.10-0318. [DOI] [PubMed] [Google Scholar]
- 40.Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ. Determinants of ventilatory efficiency in heart failure. Circ Heart Fail. 2008;1:227–33. doi: 10.1161/CIRCHEARTFAILURE.108.785501. https://doi.org/10.1161/CIRCHEARTFAILURE.108.785501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction: right ventricular dysfunction in heart failure. Eur J Heart Fail. 2017 doi: 10.1002/ejhf.873. https://doi.org/10.1002/ejhf.873. [DOI] [PubMed]
- 42.Dexter L. Atrial Septal Defect Heart. 1956;18:209–25. doi: 10.1136/hrt.18.2.209. https://doi.org/10.1136/hrt.18.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017;19:873–9. doi: 10.1002/ejhf.664. https://doi.org/10.1002/ejhf.664. [DOI] [PubMed] [Google Scholar]
- 44.Dini FL, Carluccio E, Simioniuc A, Biagioli P, Reboldi G, Galeotti GG, et al. Right ventricular recovery during follow-up is associated with improved survival in patients with chronic heart failure with reduced ejection fraction: right ventricular recovery in heart failure. Eur J Heart Fail. 2016;18:1462–71. doi: 10.1002/ejhf.639. https://doi.org/10.1002/ejhf.639. [DOI] [PubMed] [Google Scholar]