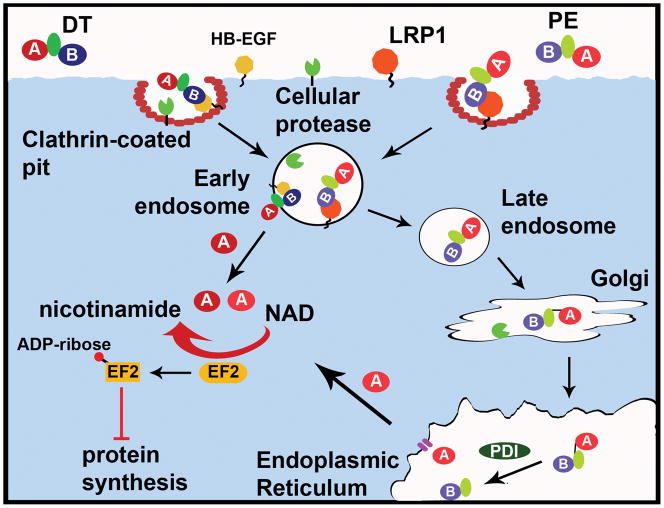
Figure 2. Diphtheria toxin-like cellular intoxication pathways.
A. Cellular intoxication of DT-like toxins. DT-like toxins bind host membrane-bound receptors and enter via receptor-mediated endocytosis. Protease cleavage of the peptide backbone results in a single disulfide-linked chain. Upon endosomal acidification, the A domain of DT is translocated into the cytosol by insertion of the translocation domain into the endosomal membrane and reduction of the disulfide bond. Conversely, PE undergoes retrograde trafficking to the ER, where reduction of the disulfide and interactions with ER-associated chaperone proteins like PDI promote translocation of the A domain through a Sec61-like channel into the cytosol. Once in the cytosol, the A domains of DT-like toxins ADP-ribosylate EF2, inhibiting protein synthesis and killing the cell. Cholix can utilize LRP1 to enter cells and may follow the PE intoxication pathway.