Figure 1.
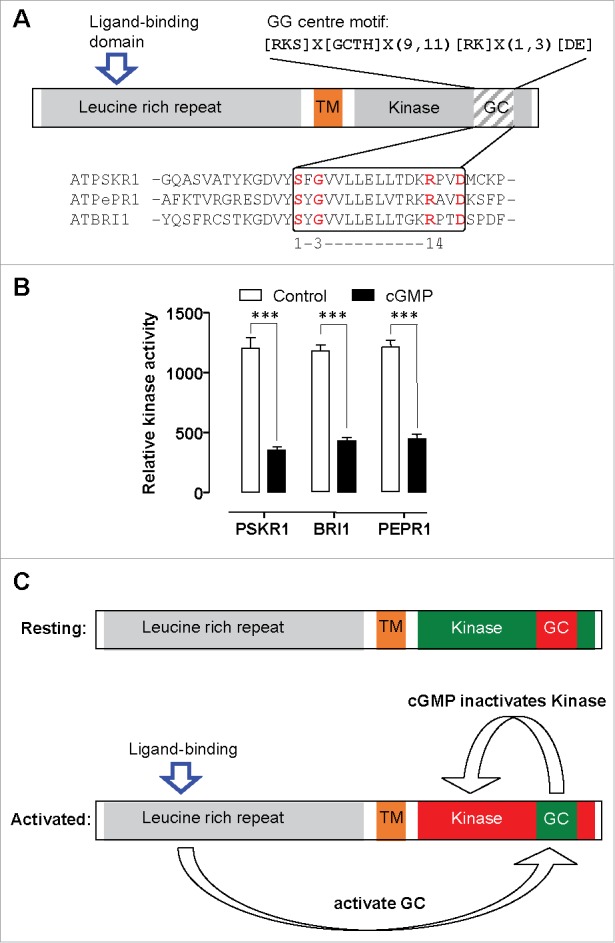
Intramolecular switching in plant receptor-like kinases. A. Schematic showing the domain architecture of receptor like kinases AtBRI1, AtPSKR1 and AtPePR1. These molecules all contain a leucine rich repeat extracellular domain that contains a ligand binding island, a transmembrane (TM) spanning domain and an intracellular kinase domain. Within the intracellular kinase domain is a guanylate cyclase (GC) catalytic center. The diagnostic GC catalytic center core motif shows the functionally assigned amino acids and these are highlighted in the alignment of this region. B. The kinase domain of AtPSKR1, AtBRI1 and AtPePR1 were recombinantly expressed in bacteria and purified using immobilized metal affinity chromatography on nickel-nitrilotriacetic acid agarose. Kinase activity was measured as an increase in fluorescence of Ser/Thr peptide 1 as a substrate in the presence or absence of 0.1 nM cGMP using the Omnia kinase assay. Data is adapted for PSKR1,4 BRI17 and from an unpublished experiment for AtPePR1 and analysed by one-way ANOVA followed by Tukey's multiple comparisons test (*** P < 0.001). C. Model showing the resting state of the receptor with active kinase that can autophosphorylate itself. Following ligand binding further phosphorylation occurs that activates guanylate cyclase center to generate cGMP which in turn inhibits kinase activity.
