ABSTRACT
TOR is the master regulator of growth and development that senses energy availability. Biotic stress perturbs metabolic and energy homeostasis, making TOR a good candidate to participate in the plant response. Fusarium graminearum (Fusarium) produces important losses in many crops all over the world. To date, the role of TOR in Fusarium infection has remained unexplored. Here, we show that the resistance to the pathogen increases in different Arabidopsis mutants impaired in TOR complex or in wild-type plants treated with a TOR inhibitor. We conclude that TOR signaling is involved in plant defense against Fusarium.
KEYWORDS: Arabidopsis, biotic stress, Fusarium graminearum, Fusarium Head Blight, TOR
The protein kinase target of rapamycin (TOR) is a master regulator that controls growth and development integrating external and internal signals (nutrients availability, hormones, etc).8,6 It is well documented that TOR signaling regulates many important biological processes such as protein translation, but it is still unclear its role in environmental stresses. In plants, TOR associates to RAPTOR (Regulatory-associated protein of TOR) and LST8 (Lethal with Sec 13) proteins to form a multiprotein complex denominated TOR COMPLEX 1. One sequence coding for a TOR protein and two for both RAPTOR (RAPTOR3G and RAPTOR5G genes, At3g08850 and At5g01770 respectively) and LST8 (LST8.1 and LST8.2 genes, At3g18140 and At2g22040 respectively) can be retrieved from the Arabidopsis genome. Despite the occurrence of two genes coding for each TOR-partner, only RAPTOR3G and LST8.1 are expressed in high levels.6 Conversely, AtRAPTOR5G is transcribed in very low levels and expression of AtLST8.2 has not been detected.1,4,11
TOR is essential to maintain the energy and metabolic homeostasis of the cells, which is extremely affected under biotic stresses. Fusarium sp. is one of the most important genera of fungal plant pathogens, causing devastating diseases in numerous economically important crops. Fusarium graminearum (Fusarium), which causes the disease Fusarium Head Blight (FHB) of wheat, barley and other cereals, is the most widespread species.15 FHB produces cereal grain yield and quality reduction, and also contamination with mycotoxins, which are dangerous for animals and even for humans. Given that, the need for investigations about signaling pathways involved in the defense response to this dangerous fungus is evident in order to develop new crop protection strategies.
In this work, we focus on the study of the role of TOR complex in the Arabidopsis thaliana-Fusarium interaction. It was described that Fusarium is able to infect the model plant Arabidopsis.14,3,13 First, we analyzed the effect of Fusarium on Arabidopsis wild-type (WT) and different TOR-complex mutant plants, TOR conditional mutant (estradiol-inducible RNA interference, herein called tor-es),16 raptor3g, lst8.1 and lst8.2. Detached-leaf infection bioassays were performed as described previously3 using cut leaves from rosette of 3-weeks-old plants. A virulent isolate of Fusarium was used to inoculate the leaves and disease severity was assessed during 7 days post inoculation (dpi). As shown in Fig. 1A-D, Fusarium infection caused important disease symptoms in WT plants, being susceptible to the fungus. However, a resistant reaction was observed in tor-es and raptor3g mutant lines, in comparison to WT plants. This is evidenced through scarcely fungus growth, presence of fewer plant dead cells (Fig. 1B), decreasing disease severity (between 5–10%, Fig. 1C) and reduction of the lesion size (Fig. 1D). The mutant line impaired in LST8.1 expression was more resistant to the fungal infection than the WT but more susceptible than tor-es and raptor3g (30% lower disease severity) (Fig. 1A-C).
Figure 1.

Arabidopsis thaliana TOR complex mutants are more resistant to Fusarium graminearum infection. (A) Disease reaction of Arabidopsis TOR complex mutants. Lesion development in WT and mutants leaves (3-weeks-old seedlings) at 7 dpi with Fusarium (5 µl of 1–5 × 105 conidia/ml suspension) (I) compared with their respective control (C). For induction of tor-RNAi of tor-es mutant, 10 µM of β-estradiol was added 24 h previous to infection. Representative images are shown. Scale bar = 2 cm. (B) Fusarium mycelial growth and plant cell death. The same leaves of (A) were stained with trypan blue reagent and visualized under stereoscope (20x). Representative images are shown. The red arrowheads indicate cell death and infectious hyphae on inoculated leaves. Scale bars = 200 µm. (C) Assessment of resistance to Fusarium infection of Arabidopsis WT and TOR complex mutants. Quantification of development disease in leaves of WT and TOR mutants plants at 7 dpi with Fusarium. Standard error bars (SE) are shown. Significant statistically differences (Tukey´s test) are indicated with asterisks (P < 0.05). (D) Lesion size measurement. Expanding lesions were measured on excised leaves at 7 dpi with Fusarium. SE are indicated. Significant statistically differences (Tukey´s test) are indicated with asterisks (p < 0.05). (E) Fusarium infection on Arabidopsis whole plants. Symptoms in Arabidopsis WT and raptor3g mutant after infection with 20 ml of Fusarium suspension (5 µl of 1–5 × 105 conidia/ml suspension) at 14 dpi. Representative images are shown. (F) Fusarium mycelial growth and plant cell death. The same leaves of (E) was stained with trypan blue reagent and visualized under stereoscope (20x). Representative images are shown. Scale bar = 200 µm. (G) Whole plant decay of (E) was measured each day of the disease assay. SE are shown.
However, the lesion sizes in the lst8.1 mutants were smaller than those presented in the WT leaves, indicating a reduction of the severity of the symptoms (Fig. 1D). On the other hand, lst8.2 mutant presented a similar infection level than WT plants, being the disease severity almost 90% (Fig. 1A-D). We also performed an infection assay in whole plant of the raptor3g mutant to corroborate the results in detached leaves. As expected, the mutant was more resistant to Fusarium infection, with a value of whole plant decay below 20% (Fig. 1E-G).
To further confirm the results, we conducted a pharmacological assay with the ATP-competitive TOR kinase inhibitor PP242.17 Arabidopsis WT plants were treated with PP242 and the symptoms and degree of infection by Fusarium were analyzed. The inhibitor treated-WT plants were less infected than the control (Fig. 2). This result demonstrates that the downregulation of TOR signaling confers more resistance/tolerance to Fusarium infection.
Figure 2.
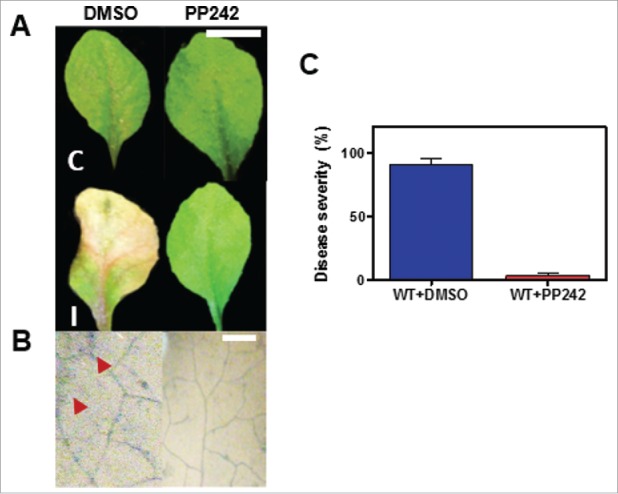
Arabidopsis WT plants with pharmacological inhibition of TOR signaling are more resistant to Fusarium infection. (A) Disease reaction of WT plants impaired in TOR signaling. Lesion development in Arabidopsis WT or WT leaves treated with the TOR inhibitor PP242 (2 µM) at 7 dpi with Fusarium (5 µl of 1–5 × 105 conidia/ml suspension) (I) compared with their respective control (C). Scale bar = 2 cm. Representative images are shown. (B) Fusarium mycelial growth and plant cell death. The same leaves of (A) were stained with trypan blue reagent and visualized under stereoscope (20x). The red arrowheads indicate cell death and infectious hyphae on inoculated leaves. Scale bars = 200 µm. (C) Assessment of resistance to Fusarium infection in pharmacological TOR-inhibited Arabidopsis WT leaves. Quantification of disease development of WT or PP242-treated WT leaves (2 µM) at 7 dpi with Fusarium. Standard error bars (SE) are indicated.
In summary, we demonstrated that TOR signaling may be involved in Arabidopsis-Fusarium interaction, using both TOR complex-proteins mutants and a second generation TOR inhibitor (PP242). The tor-es and raptor3g mutants were more resistant genotypes to Fusarium infection than Arabidopsis WT plants. One explanation of the results obtained with the TOR complex mutants is that plants behave as constitutively “primed” against pathogen. Priming is defined as an induced state whereby a plant responds more rapidly and/or more efficiently to a stress.2 In accordance with the mutant behavior against Fusarium infection, Arabidopsis WT plants treated with the TOR kinase inhibitor, PP242, were almost totally resistant to the fungus attack. Previously, it was reported that TOR signaling has a role for the potyvirus infection in Arabidopsis and that the inhibition of TOR activity prevents the disease.12 During the writing of this manuscript, Meteignier et al. (2017) and De Vleesschauwer et al. (2018)10,5 have described that TOR mutants could enhance resistance to different pathogens in Arabidopsis and in rice respectively. Our results are in agreement with both reports, contributing with an extremely important example to the idea that the role of TOR in the plant response to pathogens could be general.
Finally, our data demonstrate that TOR complex is involved in the Arabidopsis response against the fungus Fusarium which causes numerous losses of cereal crops. These results contribute to the knowledge of the mechanisms that are involved in plant defense responses, which are indispensable for biotechnology purposes. The fact that affecting RAPTOR and LST8.1 protein levels also increases the resistance to Fusarium broadens the possibility of manipulating the TOR pathway, without modifying TOR expression, and thus decreasing negative side effects.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana (Columbia-0 ecotype) was used as genetic background. TOR conditional mutant seeds (estradiol-inducible RNAi, tor-es) were a kind gift from Dr. Xiong.16 The other mutants of the TOR-Complex: raptor3g (SALK_022096C9), lst8.1 and lst8.2 (SALK_002459 and SALK_018605C respectively11) were obtained from SALK collection (http://signal.salk.edu/). Screening for the T-DNA knockout mutants was performed by PCR analysis (Table S1). tor-es mutant plants were treated with 10 µM of β-estradiol (E8875; SIGMA-Aldrich, USA) for induction of tor-RNAi 24 h previous to infection assays. The treatment with estradiol did not affect Fusarium infection (Fig. S1).
Plants were grown in pots containing soil mixture (3:1:1 mixture of peat moss-based mix:vermiculite:perlite) under a 16:8 h (light-dark) photoperiod at 22°C.
Disease assays
Leaves from 3-weeks-old plants were used in detached leaf infection bioassay system as described previously.3 Petioles of detached leaves were embedded into sterile water agar (0.7%) with the leaf blade not touching the agar surface. A Fusarium graminearum native isolate from INBIOTEC-FIBA collection was used for the infection experiments. For leaf inoculation assays, 5-µl drops of conidial suspension (1–5 × 105 conidia/ml) or water (control) were put onto the adaxial leaf surface. The plates were incubated under a 16:8-h light-dark cycle at 22°C (100% RH), and disease severity was assessed 24, 48, 72 h and 7 d post inoculation (dpi). Disease progression in the inoculated leaves was estimated by an average disease rating (0–4) as follow: the fraction of inoculated leaves exhibiting chlorosis covering <25% (category 1), 25 to 50% (category 2), 50 to 75% (category 3), and >75% (category 4) of leaf area was determined for each genotype. A minimum of 30 leaves of each genotype was analyzed for each experiment. Disease severity (as percentage) was calculated for each treatment according to the following formula, DS = [(Σ nRi)/(NRmax)] x 100, where n is the number of leaves with each disease rating (Ri), N is the total number of leaves of each line, and R max is the maximum disease rating (corresponding to 4).
For the infection assays of whole plants (4-weeks old), 20 ml of Fusarium suspension were sprayed (1–5 × 105 conidia/ml) and disease decay was determined during 14 d according Guo and Stotz.7
Microscopical analysis of infection
Leaves were transferred to chloral hydrate solution (2.5 g/ml) for clearing, and the infection process became evident by boiling infected leaves for 4 min in trypan blue solution containing equal volumes of 1 mg/ml aqueous trypan blue, lactic acid, glycerol and water. Microscopic inspection was carried out at 7 dpi using a Nikon stereoscopy (Stereoscopic Zoom Microscope SMZ 800).
TOR inhibitor treatment
Leaves from 3-weeks-old seedlings were treated or not with 2 µM PP242 (stock solution was dissolved in DMSO; P0037 SIGMA-Aldrich) in agar plates and inoculated with Fusarium as pointed above. DMSO (0.1%) was added in plates without the inhibitor as controls. Pictures were taken at 7 dpi.
Statistical analysis
The GraphPad Prism Version 5.01 program was used for statistical analysis. One-way ANOVA was performed and the differences between groups were tested using Tukey's Multiple Comparison Test with p-value< 0.05. Three independent experiments were conducted for all the assays performed in this study.
Supplementary Material
Abbreviations
- dpi
days after inoculation
- FHB
Fusarium Head Blight
- LST8
Lethal with Sec 13
- RAPTOR
Regulatory-associated protein of TOR
- TOR
Target of Rapamycin
- WT
Wild Type.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Fundación para Investigaciones Biológicas Aplicadas (FIBA) and Universidad Nacional de Mar del Plata (UNMdP). FC, GLS and GMN are career investigators of CONICET. We thank Dr. Yan Xiong for the conditional tor-es mutant.
Author contributions
GMN conceived the project. GMN and FC designed and supervised the experiments and NA performed them. GMN wrote the manuscript with the help and advice of GLS.
References
- 1.Anderson GH, Veit B, Hanson MR. The Arabidopsis AtRaptor genes are essential for post-embryonic plant growth. BMC Biol. 2005;3:12. doi: 10.1186/1741-7007-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balmer A, Pastor V, Gamir J, Flors V, Mauch-Mani B. The ‘prime-ome’: towards a holistic approach to priming. Trends Plant Sci. 2015;20:443–452. doi: 10.1016/j.tplants.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Steed A, Harden C, Nicholson P. Characterization of Arabidopsis thaliana-Fusarium graminearum interactions and identification of variation in resistance among ecotypes. Mol Plant Pathol. 2006;7:391–403. doi: 10.1111/j.1364-3703.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- 4.Deprost D, Truong HN, Robaglia C, Meyer C. An Arabidopsis homolog of RAPTOR/KOG1 is essential for early embryo development. Biochem Biophys Res Commun. 2005;326:844–850. doi: 10.1016/j.bbrc.2004.11.117. [DOI] [PubMed] [Google Scholar]
- 5.De Vleesschauwer D, Filipe O, Hoffman G, Seifi HS, Haeck A, Canlas P, Van Bockhaven J, De Waele E, Demeestere K, Ronald P, et al.. Target of rapamycin signaling orchestrates growth-defense trade‐offs in plants. New Phytol. 2018;217:305–319. doi: 10.1111/nph.14785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobrenel T, Caldana C, Hanson J, Robaglia C, Vincentz M, Veit B, Meyer C. TOR signaling and nutrient sensing. Annu Rev Plant Biol. 2016;67:261–285. doi: 10.1146/annurev-arplant-043014-114648. [DOI] [PubMed] [Google Scholar]
- 7.Guo X, Stotz HU. Defense against Sclerotinia sclerotiorum in Arabidopsis is dependent on jasmonic acid, salicylic acid, and ethylene signaling. Mol Plant Microbe Interact. 2007;20:1384–1395. doi: 10.1094/MPMI-20-11-1384. [DOI] [PubMed] [Google Scholar]
- 8.Henriques R, Bögre L, Horváth B, Magyar Z. Balancing act: matching growth with environment by the TOR signalling pathway. J Exp Bot. 2014;65:2691–2701. doi: 10.1093/jxb/eru049. [DOI] [PubMed] [Google Scholar]
- 9.Kravchenko A, Citerne S, Jéhanno I, Bersimbaev RI, Veit B, Meyer C, Leprince AS. Mutations in the Arabidopsis Lst8 and Raptor genes encoding partners of the TOR complex, or inhibition of TOR activity decrease abscisic acid (ABA) synthesis. Biochem Biophys Res Commun. 2015;467:992–997. doi: 10.1016/j.bbrc.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Meteignier LV, El Oirdi M, Cohen M, Barff T, Matteau D, Lucier JF, Rodrigue S, Jacques PE, Yoshioka K, Moffett P. Translatome analysis of an NB-LRR immune response identifies important contributors to plant immunity in Arabidopsis. J Exp Bot. 2017;68:2333–2344. doi: 10.1093/jxb/erx078. [DOI] [PubMed] [Google Scholar]
- 11.Moreau M, Azzopardi M, Clément G, Dobrenel T, Marchive C, Renne C, Martin-Magniette ML, Taconnat L, Renou JP, Robaglia C, et al.. Mutations in the Arabidopsis homolog of LST8/GβL, a partner of the target of rapamycin kinase, impair plant growth, flowering, and metabolic adaptation to long days. Plant Cell. 2012;24:463–481. doi: 10.1105/tpc.111.091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouibrahim L, Rubio AG, Moretti A, Montané MH, Menand B, Meyer C, Robaglia C, Caranta C. Potyviruses differ in their requirement for TOR signalling. J Gen Virol. 2015;96:2898–2903. doi: 10.1099/vir.0.000186. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber KJ, Nasmith CG, Allard G, Singh J, Subramaniam R, Desveaux D. Found in translation: high-throughput chemical screening in Arabidopsis thaliana identifies small molecules that reduce Fusarium head blight disease in wheat. Mol Plant Microbe Interact. 2011;24:640–648. doi: 10.1094/MPMI-09-10-0210. [DOI] [PubMed] [Google Scholar]
- 14.Skadsen RW, Hohn TM. Use of Fusarium graminearum transformed with gfp to follow infection patterns in barley and Arabidopsis. Physiol Mol Plant Pathol. 2004;64:45–53 doi: 10.1016/j.pmpp.2004.04.003. [DOI] [Google Scholar]
- 15.Yang F, Jacobsen S, Jørgensen HJ, Collinge DB, Svensson B, Finnie C. Fusarium graminearum and its interactions with cereal heads: studies in the proteomics era. Front Plant Sci. 2013;4:37. doi: 10.3389/fpls.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong Y, Sheen J. Rapamycin and glucose-target of rapamycin (TOR) protein signaling in plants. J Biol Chem. 2012;287:2836–2842. doi: 10.1074/jbc.M111.300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong Y, Sheen J. Novel links in the plant TOR kinase signaling network. Curr Opin Plant Biol. 2015;28:83–91. doi: 10.1016/j.pbi.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


