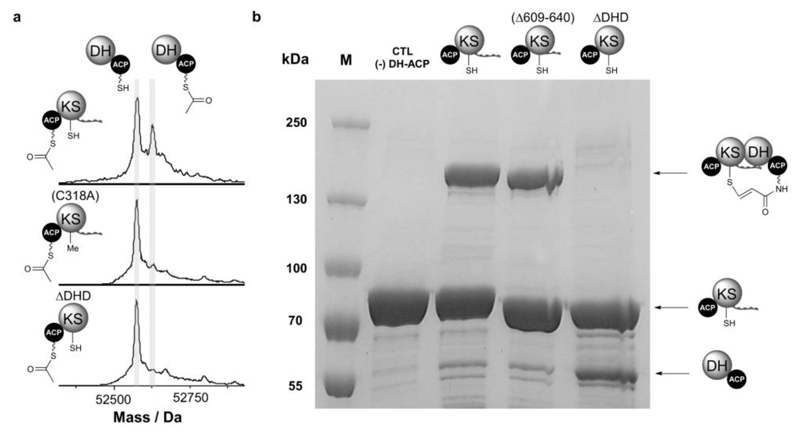
Figure 2. Acyl transfer and protein crosslinking assays demonstrate that DHD domains play a key role in communication across KS/DH interfaces.
(a) Deconvoluted mass spectra of GbnD5 holo-DH-ACP, following incubation with GbnD4 Ac-ACP-KS-DHD (top), Ac-ACP-KS(C318A)-DHD (middle) and Ac-ACP-KS(ΔDHD) (bottom). Transfer of the acetyl group onto the holo-DH-ACP di-domain results in a +42 Da mass shift. The data show that an acyl group can be transferred across the subunit interface (top), and that the KS active site Cys residue plays an important role in this process (middle). No acetylation of the holo- DH-ACP di-domain is observed when the DHD domain is removed (bottom).
(b) SDS-PAGE (6%) analysis of crosslinking reactions between the GbnD5 DH-ACP di-domain loaded with the chloroacrylamide-terminated ppant analogue and the GbnD4 ACP-KS-DHD tri-domain (left), GbnD4 ACP-KS-DHD(Δ609-640) truncated tri-domain (middle) and the ACP-KS(ΔDHD) di-domain (right). Efficient formation of a crosslinked complex (˜130 kDa) is observed for the ACP-KS-DHD tri-domain (left) and GbnD4 ACP-KS-DHD(Δ609-640) truncated tri-domain (middle), but not the ACP-KS(ΔDHD) di-domain (right).