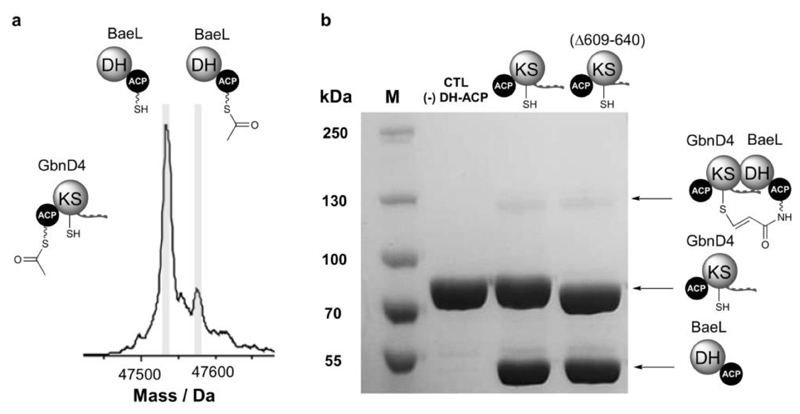
Figure 5. DHD domains interact selectively with their cognate DH domain partners.
(a) Deconvoluted mass spectrum of BaeL holo-DH-ACP di-domain following incubation with GbnD4 Ac-ACP-KS-DHD tri-domain. In comparison with the GbnD4 holo-DH-ACP di-domain, only low levels of acetylation can be observed, suggesting that the GbnD4 DHD and BaeL DH domains interact weakly.
(b) SDS-PAGE (6%) analysis of the crosslinking reaction between GbnD4 ACP-KS-DHD tri-domain and GbnD4 ACP-KS-DHD(Δ609-640) truncated tri-domain with BaeL DH-ACP di-domain loaded with the β-chloroacrylamido ppant analogue. Only trace amounts of the crosslinked complex (˜130 kDa) can be observed, providing further evidence for a weak interaction between non-cognate DHD and DH domains.