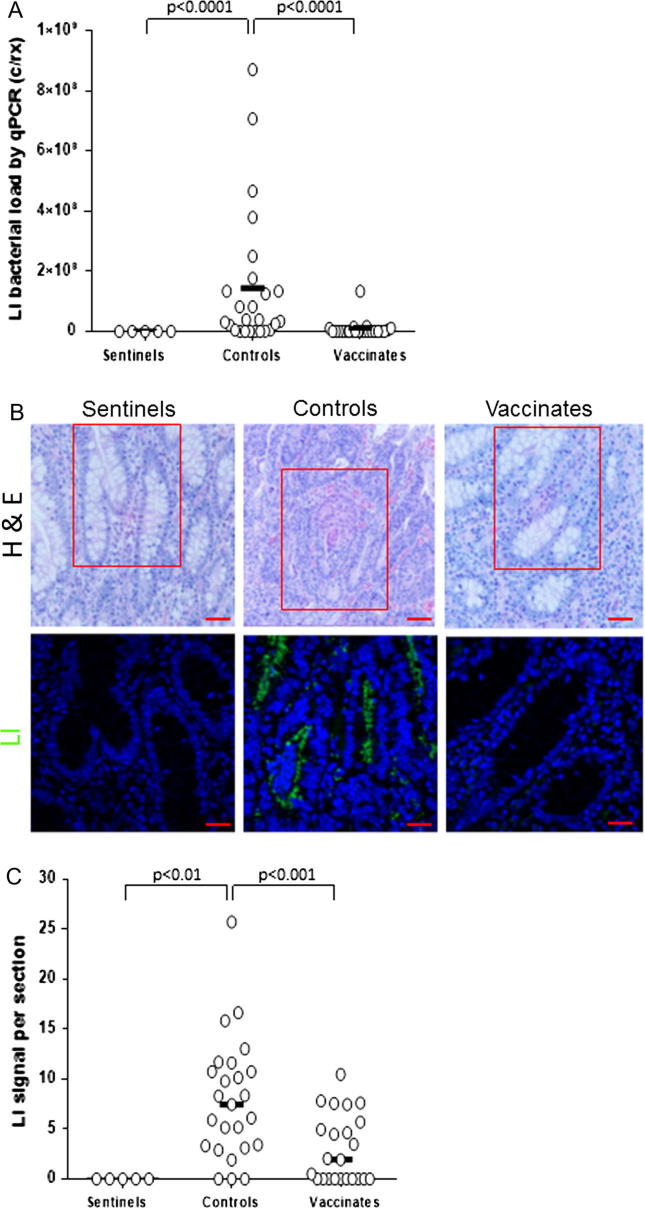
Fig. 3.
Quantification of L. intracellularis infection using qPCR and immunofluorescence. (A) qPCR was conducted to determine the amount of L. intracellularis DNA in mucosal scrapings collected at 21 days post challenge. qPCR results were reported as threshold cycle numbers (Ct) and were converted to copies per reaction (c/rx) as described in the text. Assays were concluded after 40 cycles. Group mean values are shown, and p-values when p < .05. (B) H&E and IF detection of L. intracellularis bacteria. H&E and IF assays were conducted in a blinded fashion on parallel sections. IF results using monoclonal VPM53 antibody, showing L. intracellularis bacteria (LI) in representative sentinel, control and vaccinate ileum samples 21 dpc. The presence of anti L. intracellularis antibodies (VPM53) was detected using FITC-conjugated secondary antibodies (green). Nuclei were counterstained with DAPI (Blue). Red box in H&E represents the same location on parallel section used to detect L. intracellularis bacterium. Scale bar (red line): 50 µm for H&E and 20 µm for LI. Signal intensity was determined as described in materials and methods section. (C) Scatterplot of L. intracellularis staining signal intensity obtained using Image J software for sentinel, control and vaccinate pig ileum crypts at 21 dpc. For statistical analysis between groups a 1-way ANOVA was used. Group medians are shown, and p-values where statistically significant differences were found (p < .05). Y-axis represents fluorescence signal intensity of L. intracellularis antigen staining per section. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article, Supplementary Table 1.)