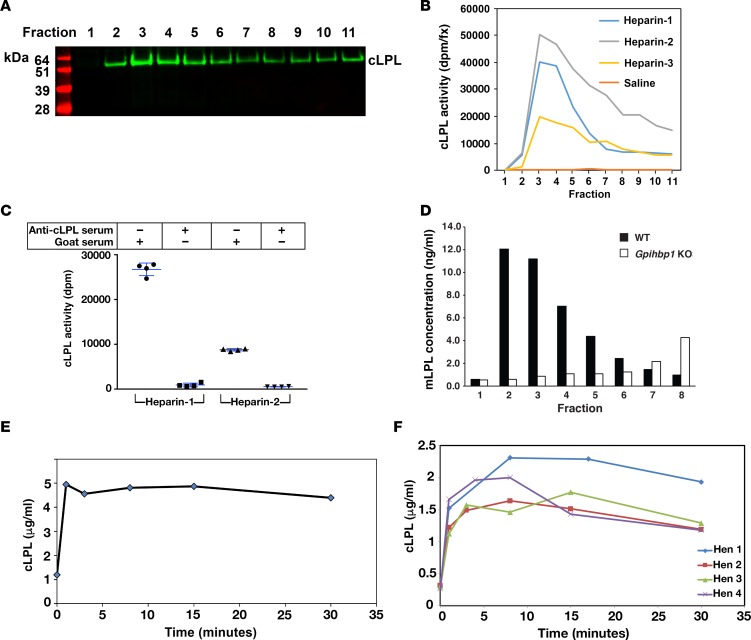
Figure 5. Chicken lipoprotein lipase (cLPL) can be released from tissues with heparin.
(A) Isolated chicken hearts were perfused with 20 U/ml heparin, and cLPL protein was detected in individual fractions (0.2 ml/fraction) by Western blotting with a goat antibody against cLPL. (B) cLPL activity in the fractions from 3 different chickens (Heparin-1, Heparin-2, Heparin-3) was measured with a [3H]triolein substrate. As a control, a chicken heart was perfused with saline only (Saline). (C) Inhibition of the LPL activity with a goat antiserum against cLPL. For these studies, we pooled fractions 3–5 from 2 of the chickens (Heparin-1 and Heparin-2). We aliquoted 25 μl of the pooled fractions and then added either 50 μl of the goat antiserum against cLPL or normal goat serum. We then performed LPL activity assays. The 4 data points represent duplicate lipase assays on the fractions from 2 chickens. (D) Bar graph showing rapid heparin-mediated release of mouse LPL (mLPL) from an isolated heart of a wild-type mouse (black bars). Heparin-mediated release of mLPL from the heart of a Gpihbp1-deficient mouse was delayed (white bars). Similar results were observed in 2 other pairs of wild-type and Gpihbp1-deficient mice. (E) Rapid release of cLPL (as measured by ELISA) into the plasma of a cockerel after an intravenous injection of heparin (2 U/g body weight). (F) Rapid release of cLPL into the plasma of 4 hens after an intravenous injection of heparin (2 U/g body weight).