Figure 8.
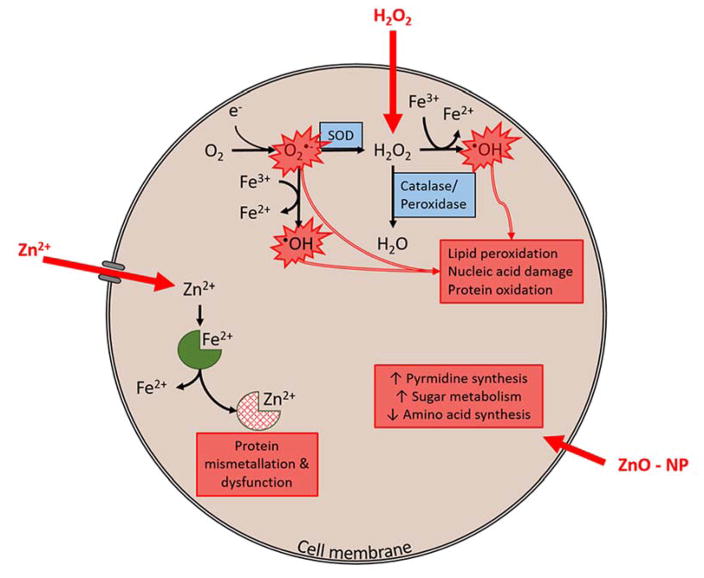
H2O2 readily diffuses through the cell membrane. High concentrations overwhelm catalase and peroxidase enzymes leading to hydroxyl radical formation via Fenton reaction. In addition, high concentration of H2O2 can inhibit superoxide dismutase (SOD) driving Haber-Weiss reaction and additional production of hydroxyl radical. These ROS (hydroxyl radical and superoxide radical) cause toxicity through lipid peroxidation, nucleic acid damage and protein oxidation. Zn2+ ions require cell membrane transporters to enter the cell. In high concentrations that exceed normal homeostatic mechanism these ions displace other metal ions cofactors on proteins, causing mis-metallation and resulting protein dysfunction. Evidence here suggests an alternative mechanism for ZnO-NPs. ZnO-NPs result in dramatic increases in pyrimidine biosynthesis, sugar metabolism and decreases in amino acid synthesis. This combination or cellular processes suggests that ZnO-NPs alter energy metabolism within the cell. The precise mechanism by which ZnO-NPs enter the cell and the specific intracellular molecular targets remain unclear.