Abstract
Purpose
After a cerebrovascular accident (CVA) aerobic deconditioning contributes to diminished physical function. Functional Electrical Stimulation (FES)-assisted cycling is a promising exercise paradigm designed to target both aerobic capacity and locomotor function. This pilot study aimed to evaluate the effects of an FES-assisted cycling intervention on aerobic capacity and locomotor function in individuals post-CVA.
Methods
Eleven individuals with chronic (> 6 months) post-CVA hemiparesis completed an 8-week (3x/wk; 24 sessions) progressive FES-assisted cycling intervention. VO2peak, self-selected and fastest comfortable walking speeds, gait and pedaling symmetry, 6-minute walk test (6MWT), balance, dynamic gait movements, and health status were measured at baseline and post-training.
Results
FES-assisted cycling significantly improved VO2peak (12%, p=0.006), self-selected walking speed (SSWS, 0.05±0.1 m/s, p=0.04), Activities-specific Balance Confidence scale score (12.75±17.4, p=0.04), Berg Balance Scale score (3.91±4.2, p=0.016), Dynamic Gait Index score (1.64±1.4, p=0.016), and Stroke Impact Scale participation/role domain score (12.74±16.7, p=0.027). Additionally, pedaling symmetry, represented by the paretic limb contribution to pedaling (PPR), significantly improved (10.09 ± 9.0%, p=0.016). Although step length symmetry (PSR) did improve, these changes were not statistically significant (−0.05±0.1%, p=0.09). Exploratory correlations showed moderate association between change in SSWS and 6MWT (r=0.74) and moderate/stroke negative association between change in PPR and PSR (r= −0.63).
Conclusion
These results support FES-assisted cycling as a means to improve both aerobic capacity and locomotor function. Improvements in SSWS, balance, dynamic walking movements, and participation in familial and societal roles are important targets for rehabilitation of individuals following CVA. Interestingly, the correlation between PSR and PPR suggests that improvements in pedaling symmetry may translate to a more symmetric gait pattern.
Keywords: Exercise, Cycling, Gait, CVA
INTRODUCTION
Cardiorespiratory fitness is reduced by as much as 50% after a cerebrovascular accident (CVA) when compared to age-matched sedentary individuals (1). Reduced aerobic capacity can negatively impact other physiological processes that result in changes in muscular and molecular abnormalities, impaired hemodynamic responses, and altered metabolic health (2,3). These changes are further associated with clinically relevant residual deficits in walking as well as an increased dependence for activities of daily living (3,4). In all, these impairments result in reduced quality of life and place individuals at increased risk for a recurrent CVA, comorbidities (e.g. cardiovascular disease), and mortality (5).
A primary objective in post-CVA rehabilitation is to improve walking capacity and gait-related activities, however, most rehabilitation therapy targeting locomotor function employs exercise well below aerobic intensities necessary for improving cardiorespiratory fitness (6,7). Research has shown that aerobic capacity is correlated with walking speed and distance, suggesting the importance of a training modality that targets improving aerobic capacity (8).
The theory behind interventions designed to improve locomotor outcomes is centered around the repetitive nature of the movements, which is thought to be a driving force behind functional recovery (9,10,11). One training modality that has not received as much attention in individuals with chronic CVA is the use of stationary cycling, with or without functional electrical stimulation (FES) assistance (12,13). Cycling generates reciprocal repetitive movements that require properly timed intra- and inter-muscular coordinated activation between the flexors and extensors of the hip, knee, and ankle which is also required during walking (11). Cycling can not only improve aerobic capacity in individuals post-CVA but the kinematics during pedaling have been shown to be similar to walking. Thus, cycling may be a way to use a non-task specific exercise to positively impact a task specific activity (i.e. walking) (14).
Even though there is general agreement regarding the overall benefits of exercise after a CVA, wide differences in training intensity, frequency, and type of activity (walking, cycling, mixed training) make it difficult to establish the respective impact on cardiovascular fitness and locomotor function in individuals post-CVA (15). FES-assisted cycling has been shown to increase muscle strength, activate paretic muscles, increase aerobic capacity, and improve pedaling symmetry and smoothness (12,16,17,18). Additionally, several studies have shown that FES cycling supports rehabilitation of postural control and walking function (12,13,16,17). These studies, however, only investigated single session changes, included patients in the acute to subacute phase post-CVA, or used passive cycling (12,13,16,17,18,19).
The primary objective of this pilot study, therefore, was to determine the effects of an FES-assisted active cycling intervention on aerobic capacity and locomotor function in individuals with chronic CVA. A secondary objective was to explore correlations between change scores in primary outcomes of aerobic capacity (VO2peak), symmetry (pedaling and gait), and locomotor function. Additionally, clinical measures of balance (static and dynamic), speeds and distances walked, as well as individual’s perceived health-related quality of life were also assessed.
METHODS
Participants
Thirteen individuals with post-CVA hemiparesis were enrolled in an 8-week (3x/wk; 24 sessions) FES-assisted cycling intervention. Inclusion criteria were: 1) age 18–70, 2) time since CVA ≥ 6 months, 3) residual lower extremity paresis (LE Fugl-Meyer <34), and 4) self-selected gait speed <1.00 m/s. In addition, all subjects who met selection criteria were cleared for participation following completion of an exercise tolerance test overseen by a cardiologist. This graded exercise tolerance test was performed without FES assistance and therefore a completely voluntary effort by the individual. Exclusion criteria were: 1) intermittent claudication while walking <200 m, 2) history of significant cardiac disease, angina or dyspnea at rest or during ADL’s, 3) dementia or pre-existing neurological disorders other than CVA, 4) history of major head trauma, 5) legal blindness or severe visual impairment, 6) history of significant psychiatric illness, 7) severe arthritis or other problems that limit passive range of motion, 8) severe hypertension (systolic >200 mmHg and diastolic >110 mmHg at rest), and 9) presence of a cardiac pacemaker or other contraindications to FES.
Written informed consent was obtained from all subjects prior to participation and all aspects of this study were performed in accordance with the protocol approved by the Institutional Review Board at the Medical University of South Carolina (MUSC), Charleston, South Carolina.
FES-Assisted Cycling Intervention
A recumbent tricycle was equipped with a FES system that assisted the individual only if pedaling power output was not sufficient to meet the desired workload determined for each session (Figure 1). The FES cycling system consisted of a commercially available recumbent sport tricycle (KMX Karts, UK. http://www.kmxkarts.co.uk) and a RehaStim stimulator (HasomedGmbH, Magdeburg, Germany). Stimulating electrodes were placed over the proximal-lateral and distal-medial aspects of the quadriceps muscle group of both legs. Stimulation intensity was controlled by a custom software program developed in MATLAB (6.1, The MatchWorks Inc., Natck, MA, 2000) and used to set the stimulation intensity threshold for each individual as well as the timing of stimulation onset. This software allows setting of a target power output (product of pedal cadence and resistance) for each cycling session with instantaneous feedback, thus enabling the program to adjust the level of stimulation to attain and maintain the targeted session intensity as needed. The software program received information from an encoder on the pedal crank that detected position and provided stimulation at the appropriate time to elicit activation of the quadriceps femoris muscle group at the correct time during the pedal stroke. Stimulation max intensity was determined before the first session for each individual and adjusted to tolerance by having the individual pedal below desired power output allowing the system to turn on the stimulation. Intensity was increased incrementally to a level that study staff could see the quadriceps contract and still tolerable by the individual. During training, stimulation was triggered to assist the subject if power output was not maintained and shut off when participant volitional effort was able to meet or exceed targeted levels. The percent of max stimulation intensity delivered was dependent on how far voluntary efforts fell below the targeted power output (i.e. 10% below target level stimulation was less than if 25% below power output goal). A manuscript describing the system in detail has been published by McRae et al., 2009 (20). An emergency stop switch controlled by the investigators or subject could override the delivery of stimulation at any point. Additionally, to assist individuals in reaching the targeted exercise intensity required for a given session, visual feedback was provided in the form of a target box representing desired work rate projected onto a screen and a moving cursor that represented the individual’s actual cadence. Individuals were instructed to pedal at a pace that kept the moving cursor inside the target box. Pedal cadence and resistance were adjusted to achieve the desired intensity; however, the focus of progression during training was primarily on increasing cadence as opposed to pedal resistance. Visual feedback was also controlled by the custom MATLAB program.
Figure 1.
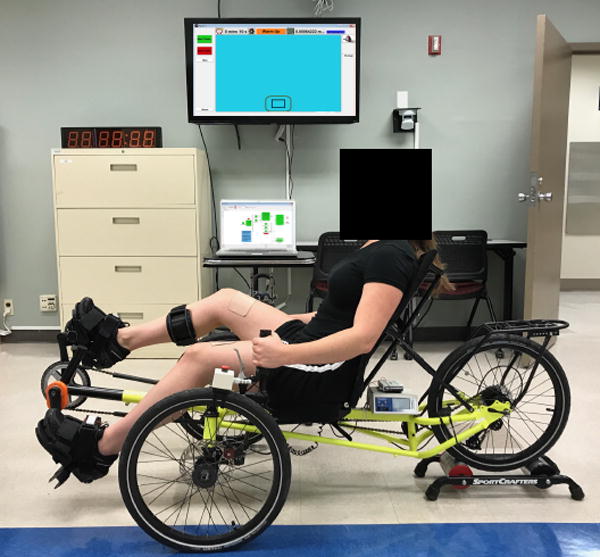
Set up of recumbent bike, software, and visual feedback for exercise training sessions.
Exercise sessions were conducted in the locomotor rehabilitation research laboratory located on MUSC’s campus. Subjects exercised for 30 minutes with rest breaks as needed. Resting and max heart rate determined during the pre-intervention exercise tolerance test were used to calculate HRR so as to determine required weekly exercise intensities. In some individuals, a higher heart rate occurred during the training intervention sessions and therefore HRR was adjusted to ensure exercising at proper intensity. Week one intensity was 40–50% HRR and progressed to 70–80% HRR by end of week eight; approximately 10% increase in intensity every two weeks. This training intervention was novel in that the program design included alternating steady state and interval training sessions, with total “work” consistent between alternating conditions to maintain the desired intensity (%HRR) for the week. For example, week one had a target intensity of 40% HRR so the steady state session heart rate was kept around this target and the interval training session was three minutes at 60% HRR followed by three minutes at 20% HRR. Week eight had a target intensity of 75% HRR for steady state sessions and the interval training sessions were three minutes of as close as possible to 100% HRR followed by three minutes at 20% HRR.
Heart rate and blood pressure were monitored prior to, during, and following completion of each training session. Blood pressure must have been below 200 mmHg for systolic and 100 for diastolic, as well as a heart rate below 110 beats per minute, to begin the session. Sessions were terminated if excessive blood pressure changes occurred by systolic blood pressure >200 mmHg, diastolic blood pressure >110 mmHg, a drop in systolic blood pressure of >10 mmHg, or inappropriate bradycardia (>15 beats per minute).
Metabolic Capacity
The pre-intervention exercise tolerance test, which was the session test overseen by the study cardiologist for inclusion into the study, involved a cycling graded exercise tolerance test protocol with heart rate and metabolic capacity (VO2peak) recorded during the session. The exercise tolerance testing was completed in the same laboratory and on the same recumbent bike as the exercise intervention sessions. A facemask was worn during testing that allowed for continuous measurement of metabolic gas exchange (COSMED Quark CPET, Rome, Italy). Testing began with the subject pedaling at approximately 40 revolutions per minute with workload, controlled through cadence or resistance, increased slightly every three minutes. Testing continued until maximum effort was achieved or testing was terminated for predefined symptomatic, clinical, and electrocardiographic criteria. The length of each individuals test differed as some individuals were more fit than others but test length ranged from nine to eighteen minutes. Symptom related reasons for termination included angina, dyspnea, and fatigue and clinical criteria for termination included blood pressure > 220/120 mmHg or a drop in diastolic blood pressure >20 mmHg. Heart rate and blood pressure readings were obtained every three minutes. VO2peak was determined by averaging 30 seconds of highest oxygen value with careful attention not to include obscure values due to talking. FES was not used during the exercise tolerance test and was a purely voluntary effort.
Overground Gait Measures
Overground self-selected walking speed (SSWS), fastest comfortable walking speed (FCWS), and spatiotemporal parameters of walking were measured over a 20 ft. long gait mat (GAITRite, CIR System Inc; Sparta, New Jersey) (21). Self-selected walking speed is considered to be the normal walking pace for an individual and fastest walking speed is the fastest speed that the individual can safely walk. Individuals performed three trials at each speed with data averaged for analyses. If individuals used a cane or walker during the pre-training data collection then the same assistive device was used for post-training data collection. Paretic step ratio (PSR), calculated from the GAITRite output, is a biomechanical marker that represents the percentage of stride length accounted for by the paretic step and was used to assess walking-specific motor control and is expressed as a percentage. PSR is calculated by taking paretic step length divided by paretic step length plus non-paretic step length (22,23). PSR is reported as the absolute value of the deviation from the symmetrical value of 0.50 (23).
Pedaling Symmetry
Cycling involves reciprocal and repetitive movements similar to walking (11,14), therefore it is possible that training at higher intensities (i.e. increased cadence and resistance) will result in more contribution of the paretic limb to keep pace and translate to a more symmetric locomotor gait pattern. Paretic Pedaling Ratio (PPR), calculated from the Garmin dual-sensing pedal-based cycling power meter (Vector 2S, Garmin Ltd.), represents the percentage of paretic limb contribution to power output during cycling.
Clinical Measures
The Activities-specific Balance Confidence scale (ABC) is a 16-item self-report measure of confidence in performing various ambulatory activities. Items are rated on a scale from 0 to 100 with higher scores representing greater confidence (24). Balance ability was tested using the Berg Balance Scale (BBS), which includes 14 functional balance items with scores ranging from 0 to 56 points; better balance was indicated by a higher score (25). Fall Efficacy Scale (FallES) is a 10-item questionnaire designed to assess confidence in one’s ability to perform 10 daily tasks without falling and is used as an indicator of how someone’s fear of falling impacts physical performance. Items are rated from 1 (“very confident”) to 10 (“not confident at all”). Total scores range from 10 to 100; lower scores indicate more confidence (26). The Dynamic Gait Index (DGI) assessed dynamic gait, balance, and fall risk with 8 functional walking tasks; higher scores indicating greater function and a score of <19 indicating an increased fall risk (27). The six-minute walk test (6MWT) was initially designed to evaluate functional capacity, endurance, and exercise tolerance (28). CVA specific impairments, however, such as diminished motor control, may influence walking function to a greater extent than cardiovascular fitness in people that have had a CVA and has been shown to be sensitive to change as a result of rehabilitation interventions targeting walking performance (29, 30,31,32). Improvement in walking distance has been observed to concur with improvement in comfortable and maximum walking speed and is being used in this study as a way to assess walking performance (31). The perceived impact of CVA was measured using the 59-item Stroke Impact Scale (SIS), which is a self-report instrument developed to assess the individual’s perception on physical aspects and dimensions of health-related quality of life (33). Emotion, communication, memory and thinking, social role, as well as physical function perception were assessed in eight domains. Scores were generated for each domain using the required equation with higher scores indicating higher physical function and health-related quality of life. Additionally, a single question on perceived recovery, which asked how much the individual feels that he/she has recovered from his/her CVA, was scored on a scale from 0–100.
Clinical measures were collected over a two-day period with exercise tolerance test and 6MWT on different days. Testing duration for each day was one to two hours. Overground SSWS and clinical measures were assessed before 6MWT or exercise tolerance test to ensure exercise induced fatigue during these demanding tasks did not influence results. The exercise tolerance test, overground SSWS and FCWS, as well as clinical assessments were performed in the same academic research laboratory as the training intervention. 6MWT was performed in a circular hallway that allowed for continuous walking. Data collections were completed within the week immediately before and after the intervention. Set-up time for a single exercise session took approximately fifteen minutes which included getting the individual onto the recumbent bike, assessing resting vital signs, placing the FES electrodes on the quadriceps, and ensuring the RehabStim system and visual feedback were ready. From start to finish the session lasted approximately one hour.
ANALYSIS
This study utilized a within-subject pre/post design. SAS version 9.4 (SAS Institute, Inc., Cary, NC) was used for statistical analyses. For continuous variables (VO2peak, SSWS, FCWS, 6MWT, PSR, and PPR) paired sample t-tests compared baseline to post-training scores. For discrete variables (ABC, BBS, DGI, FallES and SIS) the Wilcoxon signed rank test compared baseline to post-training scores. Exploratory correlations between changes in primary continuous variables from baseline to post-training were determined using Pearson’s correlational coefficients.
RESULTS
Participants
No adverse effects of training were reported. One subject stopped training two weeks early due to a health event unrelated to training, but did not report any adverse effects or negative perceptions of training. This individual completed all post-training assessments and was included in analysis as determined a priori. Two subjects were not included in analysis due to one subject being withdrawn from the study due to non-compliance and the other individual had a baseline SSWS >1.00 (1.27 m/sec). Therefore, eleven subjects were included in analysis (Table 1).
Table 1.
Individual subject characteristics as well as SSWS and aerobic capacity pre- and post-training values.
| Subject | Age (yrs) | Gender | Type | Time (mos.) | Paretic Side | Assistive Device | SSWS Pre | SSWS Post | VO2peak Pre | VO2peak Post |
|---|---|---|---|---|---|---|---|---|---|---|
| 001 | 57 | M | H | 47 | L | QC | 0.27 | 0.33 | 18.6 | 19.3 |
| 002 | 27 | F | H | 27 | L | None | 0.41 | 0.40 | 17.4 | 19.0 |
| 003 | 59 | F | I | 30 | R | None | 0.18 | 0.24 | 12.5 | 15.0 |
| 004 | 65 | M | H | 27 | R | RW | 0.36 | 0.36 | 13.2 | 11.1 |
| 005 | 63 | F | I | 25 | L | QC | 0.28 | 0.31 | 17.0 | 18.8 |
| 006 | 60 | F | I | 50 | R | None | 0.17 | 0.27 | 14.7 | 15.9 |
| 007 | 65 | F | I | 21 | R | None | 0.59 | 0.70 | 24.2 | 25.2 |
| 008 | 67 | F | I | 8 | L | RW | 0.08 | 0.15 | 16.2 | 21.3 |
| 009 | 32 | F | H | 92 | L | QC | 0.23 | 0.29 | 14.5 | 16.2 |
| 010 | 56 | M | I | 15 | R | RW | 0.40 | 0.36 | 18.7 | 24.2 |
| 011 | 46 | F | I | 13 | R | None | 0.87 | 1.00 | 12.5 | 14.5 |
Type: H, Hemorrhagic; I, Ischemic. Assistive Device: QC, quad cane; RW, rolling walker. SSWS, self-selected walking speed in m/sec. VO2peak, aerobic capacity in ml/kg/min.
Outcome Measures
VO2peak significantly improved 1.93±2.1 ml/min/kg (p=0.006) from baseline to post-training, which resulted in a 12±13.1 % improvement as a group. SSWS significantly improved 0.06±0.02 m/s (p=0.04) from baseline to post-training, however FCWS did not show a significant change (p=0.87). PPR significantly improved 10.09±9.0 % (p=0.016), however PSR did not reach significance (p=0.09). ABC significantly improved 12.75±17.4 points (p=0.04), BBS significantly improved 3.91±4.2 points (p=0.016), DGI significantly improved 1.64±1.4 points (p=0.016), and SIS participation/role domain significantly improved 12.74±16.7 points (p=0.027) from baseline to post-training. There were no significant changes for FallES, 6MWT, or the other domains and recovery question on the SIS (Table 2).
Table 2.
Pre- and Post-training assessment scores (mean±SD).
| Baseline | Post-Training | |
|---|---|---|
| ABC | 44.69 ± 19.8 | 57.45 ± 17.8 |
| BBS | 36.09 ± 11.0 | 40.00 ± 8.4 |
| DGI | 11.18 ± 3.7 | 12.82 ± 4.0 |
| FallES | 25.64 ± 12.9 | 27.64 ± 16.5 |
| SIS | ||
| Strength | 23.64 ± 10.0 | 29.09 ± 13.4 |
| Memory/Thinking | 60.52 ± 11.9 | 62.86 ± 14.7 |
| Emotion | 54.14 ± 8.5 | 54.14 ± 13.4 |
| Communication | 67.79 ± 11.9 | 70.13 ± 13.0 |
| ADL/IADL | 58.73 ± 7.1 | 55.09 ± 9.0 |
| Mobility | 51.11 ± 10.6 | 53.13 ± 11.2 |
| Hand Function | 20.73 ± 22.6 | 28.36 ± 29.3 |
| Participation/Role | 42.04 ± 15.0 | 54.77 ± 16.3 |
| Recovery | 48.18 ± 17.1 | 46.82 ± 14.2 |
| VO2peak (ml/min/kg) | 16.32 ± 3.5 | 18.23 ± 4.3 |
| SSWS (m/s) | 0.35 ± 0.2 | 0.40 ± 0.2 |
| FCWS (m/s) | 0.51 ± 0.3 | 0.51 ± 0.3 |
| 6MWT (ft.) | 456.27 ± 288.2 | 492.05 ± 343.8 |
| PSR | 0.19 ± 0.2 | 0.15 ± 0.1 |
| PPR | 16.86 ± 20.3 | 26.95 ± 22.2 |
Bold post-training assessment scores indicates statistical significance (p<0.05). ABC, Activities-specific Balance Confidence scale. BBS, Berg Balance Scale. DGI, Dynamic Gait Index. FallES, Fall Efficacy Scale. SIS, Stroke Impact Scale. SSWS, Self-selected walking speed. FCWS, Fastest comfortable walking speed. 6MWT, Six-minute walk test. PSR, Paretic step ratio. PPR, Paretic pedal ratio.
A change in SSWS had a strong positive correlation with a change in 6MWT (r=0.74; Figure 2D), however a weak negative correlation with a change in VO2peak (r = −0.10; Figure 2A). Additionally, a change in VO2peak showed no correlation with 6MWT (r = −0.07; Figure 2C). A change in PSR had a moderate to strong negative correlation with PPR (r = −0.63; Figure 2B).
Figure 2.
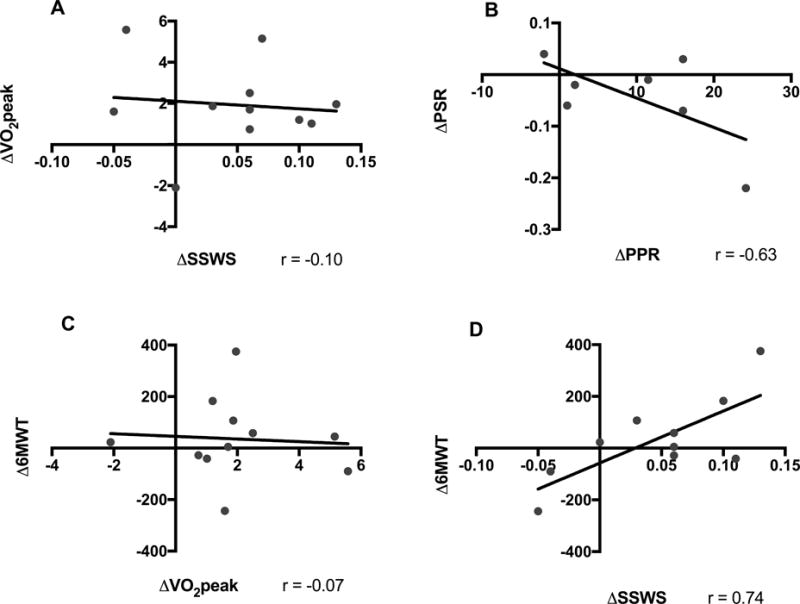
Exploratory correlations between outcomes. SSWS, Self-selected walking speed (m/sec). FCWS, Fastest comfortable walking speed (m/sec). 6MWT, Six-minute walk test (ft.). VO2peak, Peak oxygen consumption (ml/kg/min). PSR, Paretic step ratio. PPR, Paretic pedal ratio.
DISCUSSION
The results of this study show that 24 sessions of FES-assisted cycling is feasible in individuals with chronic CVA. The use of active cycling with FES assistance seems to be a promising therapeutic approach to significantly improve metabolic capacity and walking performance in individual’s post-stroke with mild to moderate hemiparesis. Additionally, clinical outcomes assessing balance, dynamic movements, and perception of participation in meaningful familial and social activities significantly improved.
To our knowledge there are only a handful of studies that have analyzed the effects of stationary cycling training on aerobic capacity (specifically VO2peak) and walking performance (e.g. walking speed, distance, PSR) in individuals with chronic CVA. It is difficult to compare results due to different training techniques such as the use of functional electrical stimulation, motorized cycles, and biofeedback (12,34,35). In the studies that used active cycling, as opposed to more passive techniques such as motorized cycling, the results of our study are similar. In the only interval based study known post CVA, Calmels et al. (2011) found a 15% increase in VO2peak after 8-weeks of intensive cycling interval-training, as well as a significant improvement in the 6MWT and knee extension and flexion peak torque, but not walking speed for the 20-meter walk test (36). However, their study’s exercise training design greatly differed from ours in that each training session had two successive phases for four minutes at 40% of maximal workload and one minute at 80% repeated six times during a 30 minute session with cadence kept consistent at 60 rpm for every session over a two month period. Jin et al. (2013) found a much larger VO2peak improvement of 27%, as well as significant improvement in 6MWT, after 8-weeks of cycling training. However, their participants trained 5 days/wk and used lower limb weights only on the paretic limb during cycling, but did not assess walking speed (14). Janseen et al. (2008) found a 14% improvement in aerobic capacity and significant improvement in 6MWT after a 6-week of training (12 sessions) intervention and utilized electrical stimulation only on the paretic limb (12). In a study by Kim et al. (2015), gait ability was measured using the timed 10-meter walk test, which showed significant improvement after 6-weeks of 5x/wk of cycling exercise. Unfortunately, this study did not assess VO2peak or spatiotemporal gait parameters (30).
There were two design novelties implemented into this training program that are different from other studies: 1) alternating between continuous and interval training and 2) using a visual cue to control desired cadence. In our study, the interval training utilized a 1:1 ratio meaning that the same amount of time spent in the high-intensity phase was spent during the low-intensity phase of the interval, therefore this design is more demanding than the only known previous interval training study by Calmels et al. (2011). Their study utilized a 1:4 ratio meaning individuals spent only a quarter of the time in the high-intensity phase than the lower-intensity phase. The results of this study show that this style of training, a more demanding interval ratio, is feasible and safe in individuals that have had a CVA and should be a consideration for future studies. Having a visual goal of cadence gave individuals a real-time indicator of how hard they were working and where they need to maintain for the training session.
An interesting finding in the current study was the correlation between changes in paretic limb contribution to pedaling and walking paretic step symmetry. Research has shown that when individuals post-CVA pedal at faster cadences and higher resistance, pedaling becomes smoother and paretic power output increases (37). Our results show that our exercise intervention, which included progressive increases in both cadence and resistance, resulted in higher paretic limb contribution during pedaling, however smoothness was not assessed and therefore changes are unknown. These results warrant further investigation into how paretic contribution during cycling impacts overground walking limb symmetry. Despite previous reports describing the impact of metabolic capacity on walking performance following CVA, the correlations between changes in VO2peak and changes in walking were not significant in our study. Although metabolic capacity may be reflected by 6MWT performance in control subjects, performance on this test in individuals following CVA is highly dependent on walking speed (38). The correlation between SSWS and 6MWT was strongly correlated in the present study (Table 2D), thus supporting the role of speed on 6MWT outcomes following CVA. There is high likelihood that improvements in metabolic capacity influence other functional as well as long-term health related outcomes and we believe that training to improve metabolic capacity should still be a focus following CVA.
There are many additional benefits to FES-assisted cycling exercise that may have a bigger impact on general health in comparison to other therapeutic modalities that do not impact cardiovascular function (e.g. biofeedback training). After a CVA, individuals remain at a high risk for another CVA with nearly a third of individuals experiencing a recurrent CVA within five years, despite optimal medical management. Furthermore, comorbid cardiovascular conditions are present in 75% of patients with CVA, representing the leading cause of death in CVA survivors. Yet, cardiovascular fitness and the modifiable characteristics of fitness training remain greatly understudied topics in this population despite the extensive evidence supporting aerobic exercise effects on modifiable risk factors relating to cardiovascular disease and CVA (39). Cycling training is an important example of a therapeutic modality that is safe, efficient, and clinically accessible and applicable that not only positively impacts physical functional recovery (e.g. ambulation, balance) but also simultaneously has a significant impact on cardiovascular function.
Limitations
The limitations of this study may affect the generalizability and applicability of the results. Most participant impairments were mild to moderate, and therefore, results of this study are not suitable to individuals with severe dysfunction. Additionally, a small sample size and no comparison group limit conclusions. Future large-scale randomized controlled clinical studies are needed to verify clinical benefits of FES-assisted cycling training on walking performance.
CONCLUSION
The present study indicates that a short 8-week (24 sessions) intensive aerobic FES-assisted cycling training intervention is a useful modality to improve cardiovascular fitness and walking speed, as well as balance, dynamic walking movements, and participation in individuals with chronic CVA.
Acknowledgments
This study was supported by NIH U54 GM104941 grant and NIH R01HD062588 grant.
Footnotes
CONFLICTS OF INTEREST
The authors have no declared conflicts of interest. The results of the study are presented clearly, honestly, and without falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
References
- 1.Mackay-Lyons MJ, Makrides L. Exercise capacity early after stroke. Arch Phys Med Rehabil. 2002;83(12):1697–702. doi: 10.1053/apmr.2002.36395. [DOI] [PubMed] [Google Scholar]
- 2.Billinger SA, Coughenour E, Mackay-Lyons MJ, Ivey FM. Reduced cardiorespiratory fitness after stroke: biological consequences and exercise-induced adaptations. Stroke Res Treat. 2012;2012:959120. doi: 10.1155/2012/959120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 4.Ivey FM, Hafer-Macko CE, Macko RF. Task-oriented treadmill exercise training in chronic hemiparetic stroke. J Rehabil Res Dev. 2008;45(2):249–59. doi: 10.1682/JRRD.2007.02.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–53. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 6.Hirschberg GG. Ambulation and self-care are goals of rehabilitation after stroke. Geriatrics. 1976;31(5):61–5. [PubMed] [Google Scholar]
- 7.MacKay-Lyons MJ, Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: is the intensity adequate to induce a training effect? Arch Phys Med Rehabil. 2002;83(10):1378–83. doi: 10.1053/apmr.2002.35089. [DOI] [PubMed] [Google Scholar]
- 8.Marzolini S, Tang A, McIlroy W, Oh PI, Brooks D. Outcomes in people after stroke attending an adapted cardiac rehabilitation exercise program: does time from stroke make a difference? J Stroke Cerebrovasc Dis. 2014;23(6):1648–56. doi: 10.1016/j.jstrokecerebrovasdis.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Del Din S, Bertoldo A, Sawacha Z, et al. Assessment of biofeedback rehabilitation in post-stroke patients combining fMRI and gait analysis: a case study. J Neuroeng Rehabil. 2014;11:53. doi: 10.1186/1743-0003-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hesse S, Werner C, Bardeleben A, Barbeau H. Body weight-supported treadmill training after stroke. Curr Atheroscler Rep. 2001;3(4):287–94. doi: 10.1007/s11883-001-0021-z. [DOI] [PubMed] [Google Scholar]
- 11.Raasch CC, Zajac FE. Locomotor strategy for pedaling: muscle groups and biomechanical functions. J Neurophysiol. 1999;82(2):515–25. doi: 10.1152/jn.1999.82.2.515. [DOI] [PubMed] [Google Scholar]
- 12.Janssen TW, Beltman JM, Elich P, et al. Effects of electric stimulation-assisted cycling training in people with chronic stroke. Arch Phys Med Rehabil. 2008;89(3):463–9. doi: 10.1016/j.apmr.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Lo HC, Hsu YC, Hsueh YH, Yeh CY. Cycling exercise with functional electrical stimulation improves postural control in stroke patients. Gait Posture. 2012;35(3):506–10. doi: 10.1016/j.gaitpost.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Jin H, Jiang Y, Wei Q, Chen L, Ma G. Effects of aerobic cycling training on cardiovascular fitness and heart rate recovery in patients with chronic stroke. NeuroRehabilitation. 2013;32(2):327–35. doi: 10.3233/NRE-130852. [DOI] [PubMed] [Google Scholar]
- 15.Marsden DL, Dunn A, Callister R, Levi CR, Spratt NJ. Characteristics of exercise training interventions to improve cardiorespiratory fitness after stroke: a systematic review with meta-analysis. Neurorehabil Neural Repair. 2013;27(9):775–88. doi: 10.1177/1545968313496329. [DOI] [PubMed] [Google Scholar]
- 16.Ambrosini E, Ferrante S, Ferrigno G, Molteni F, Pedrocchi A. Cycling induced by electrical stimulation improves muscle activation and symmetry during pedaling in hemiparetic patients. IEEE Trans Neural Syst Rehabil Eng. 2012;20(3):320–30. doi: 10.1109/TNSRE.2012.2191574. [DOI] [PubMed] [Google Scholar]
- 17.Ferrante S, Pedrocchi A, Ferrigno G, Molteni F. Cycling induced by functional electrical stimulation improves the muscular strength and the motor control of individuals with post-acute stroke. Europa Medicophysica-SIMFER 2007 Award Winner. Eur J Phys Rehabil Med. 2008;44(2):159–67. [PubMed] [Google Scholar]
- 18.Szecsi J, Krewer C, Muller F, Straube A. Functional electrical stimulation assisted cycling of patients with subacute stroke: kinetic and kinematic analysis. Clin Biomech. 2008;23(8):1086–94. doi: 10.1016/j.clinbiomech.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Bauer P, Krewer C, Golaszewski S, Koenig E, Muller F. Functional electrical stimulation-assisted active cycling–therapeutic effects in patients with hemiparesis from 7 days to 6 months after stroke: a randomized controlled pilot study. Arch Phys Med Rehabil. 2015;96(2):188–96. doi: 10.1016/j.apmr.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 20.McRae CGA, Johnston T, Lauer RT, Tokay A, Lee SCK, Hunt KJ. Cycling for children with neuromuscular impairments using electrical stimulation-development of tricycle-based systems. Med Eng Phys. 2009;31(6):650–9. doi: 10.1016/j.medengphy.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Kuys SS, Brauer SG, Ada L. Test-retest reliability of the GAITRite system in people with stroke undergoing rehabilitation. Disabil Rehabil. 2011;33(19–20):1848–53. doi: 10.3109/09638288.2010.549895. [DOI] [PubMed] [Google Scholar]
- 22.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88(1):43–9. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Bowden MG, Clark DJ, Kautz SA. Evaluation of abnormal synergy patterns poststroke: relationship of the Fugl-Meyer Assessment to hemiparetic locomotion. Neurorehabil Neural Repair. 2010;24(4):328–37. doi: 10.1177/1545968309343215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beninato M, Portney LG, Sullivan PE. Using the International Classification of Functioning, Disability and Health as a framework to examine the association between falls and clinical assessment tools in people with stroke. Phys Ther. 2009;89(8):816–25. doi: 10.2522/ptj.20080160. [DOI] [PubMed] [Google Scholar]
- 25.Flansbjer UB, Blom J, Brogardh C. The reproducibility of Berg Balance Scale and the Single-leg Stance in chronic stroke and the relationship between the two tests. PM R. 2012;4(3):165–70. doi: 10.1016/j.pmrj.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. J Gerontol. 1990;45(6):P239–43. doi: 10.1093/geronj/45.6.p239. [DOI] [PubMed] [Google Scholar]
- 27.Jonsdottir J, Cattaneo D. Reliability and validity of the dynamic gait index in persons with chronic stroke. Arch Phys Med Rehabil. 2007;88(11):1410–5. doi: 10.1016/j.apmr.2007.08.109. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–23. [PMC free article] [PubMed] [Google Scholar]
- 29.Dean CM, Richards CL, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: a randomized, controlled pilot trial. Arch Phys Med Rehabil. 2000;81(4):409–17. doi: 10.1053/mr.2000.3839. [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Cho HY, Kim YL, Lee SM. Effects of stationary cycling exercise on the balance and gait abilities of chronic stroke patients. J Phys Ther Sci. 2015;27(11):3529–31. doi: 10.1589/jpts.27.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salbach NM, Mayo NE, Wood-Dauphinee S, Hanley JA, Richards CL, Cote R. A task-orientated intervention enhances walking distance and speed in the first year post stroke: a randomized controlled trial. Clin Rehabil. 2004;18(5):509–19. doi: 10.1191/0269215504cr763oa. [DOI] [PubMed] [Google Scholar]
- 32.Tang A, Sibley KM, Bayley MT, McIlroy WE, Brooks D. Do functional walk tests reflect cardiorespiratory fitness in sub-acute stroke? J Neuroeng Rehabil. 2006;3:23. doi: 10.1186/1743-0003-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30(10):2131–40. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 34.Alon G, Conroy VM, Donner TW. Intensive training of subjects with chronic hemiparesis on a motorized cycle combined with functional electrical stimulation (FES): a feasibility and safety study. Physiother Res Int. 2011;16(2):81–91. doi: 10.1002/pri.475. [DOI] [PubMed] [Google Scholar]
- 35.Yang HC, Lee CL, Lin R, et al. Effect of biofeedback cycling training on functional recovery and walking ability of lower extremity in patients with stroke. Kaohsiung J Med Sci. 2014;30(1):35–42. doi: 10.1016/j.kjms.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Calmels P, Degache F, Courbon A, et al. The feasibility and the effects of cycloergometer interval-training on aerobic capacity and walking performance after stroke. Preliminary study Ann Phys Rehabil Med. 2011;54(1):3–15. doi: 10.1016/j.rehab.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Chen HY, Chen SC, Chen JJ, Fu LL, Wang YL. Kinesiological and kinematical analysis for stroke subjects with asymmetrical cycling movement patterns. J Electromyogr Kinesiol. 2005;15(6):587–95. doi: 10.1016/j.jelekin.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Awad LN, Reisman DS, Wright TR, Roos MA, Binder-Macleod SA. Maximum walking speed is a key determinant of long distance walking function after stroke. Top Stroke Rehabil. 2014;21(6):502–9. doi: 10.1310/tsr2106-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivey FM, Macko RF, Ryan AS, Hafer-Macko CE. Cardiovascular health and fitness after stroke. Top Stroke Rehabil. 2005;12(1):1–16. doi: 10.1310/GEEU-YRUY-VJ72-LEAR. [DOI] [PubMed] [Google Scholar]


