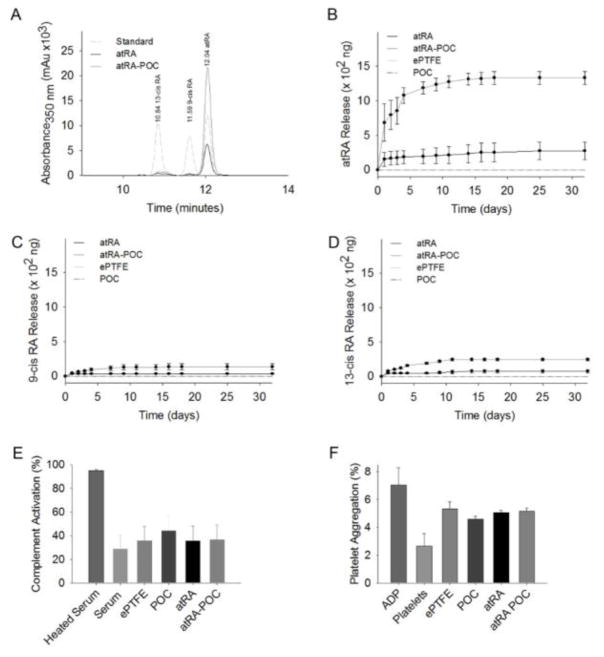
Figure 3. atRA-ePTFE and atRA-POC-ePTFE exhibit sustained release of atRA for 30 days and are biocompatible in vitro.
(A) Representative high performance chromatography (HPLC) performed on samples of retinoic acid (RA, standard), and releasate from atRA-ePTFE and atRA-POC-ePTFE. (B–D) Quantification of the isoforms of RA released from ePTFE, POC-ePTFE, atRA-ePTFE, and atRA-POC-ePTFE grafts including: (B) atRA, (C) 9-cis RA, and (D) 13-cis RA over 32 days. (E) Assessment of the percent complement activation elicited by POC-ePTFE, atRA-ePTFE, atRA-POC-ePTFE grafts as compared with ePTFE alone. (F) Assessment of the percent platelet aggregation elicited by POC-ePTFE, atRA-ePTFE, and atRA-POC-ePTFE as compared with ePTFE alone. ADP (100 μM). Data represent the mean ± SE (n=3), analysis via one-way ANOVA. ePTFE: extended polytetrafluroethylene, POC-ePTFE: poly(1,8 octamethylene citrate) ePTFE, atRA-ePTFE: all-trans retinoic acid-coated ePTFE, and atRA-POC-ePTFE: all-trans retinoic acid- poly(1,8 octamethylene citrate) ePTFE