Abstract
Partial liquid ventilation using perfluorocarbons is a therapy that was once frequently used in paediatric populations for patients with severe respiratory distress. Perfluorocarbon is a non-toxic, insoluble and radiopaque vector through which improved gas exchange can occur. Two previous cases have been reported of persistent perfluorocarbon residua, identified on imaging years after receiving liquid ventilation therapy. We report a case of perfluorocarbon detection on a CT scan 15 years after liquid ventilation at 3 months of age, and propose the probable mechanism of its appearance. The importance of considering the imaging appearances of ‘pseudo-calcifications’ as a long-term sequela to perfluorocarbon liquid ventilation is emphasised.
Keywords: neonatal and paediatric intensive care, paediatrics, neonatal intensive care, paediatric intensive care, mechanical ventilation
Background
Liquid ventilation is a technique that uses perfluorocarbon to improve gas exchange and respiratory function in patients with severe respiratory distress. Such patients include adults with acute lung injury and acute respiratory distress syndrome (ARDS), neonates with congenital diaphragmatic hernia, meconium aspiration syndrome and respiratory distress syndrome of the newborn.1 2
In total liquid ventilation, the lungs are filled with perfluorocarbon and then ventilated with liquid tidal volumes. In partial liquid ventilation (PLV) the lungs are filled with perfluorocarbon during continuous positive pressure ventilation.2 This is the more common of the two methods.
Perfluorocarbons are similar to hydrocarbons that have had hydrogen atoms replaced by fluorine. During liquid ventilation, perfluorocarbon is instilled into the lungs through an endotracheal tube while connected to a mechanical gas ventilator. Perfluorocarbon has a low surface tension and increased capacity to carry oxygen and carbon dioxide, making it a vector through which improved gas exchange can occur.3 With liquid ventilation, alveolar pressures are low because the high surface tension of the gas-lung interface is eliminated.4
The technique of PLV was commonplace in the 1990s; animal studies had modelled its use in the patient with ARDS to improve gas exchange and pulmonary blood flow, to decrease lung injury and clear lung debris.5–7 A randomised controlled trial (RCT) pilot study in humans showed a trend to decreased mortality in patients treated with PLV.8
However, in 2006, Kacmarek et al 9 published a multicentre RCT displaying that PLV did not improve outcomes when compared with a control group of conventional mechanical ventilation.
Furthermore, a 2013 Cochrane systematic review concluded that there is no evidence supporting the use of PLV in ARDS.10 A further review highlighted that currently there are insufficient data available from RCTs to support or refute the use of PLV in paediatric patients with ARDS.11
Case presentation
A 15-year-old girl with asthma reported a chronic cough productive of white sputum.
Known to have had chronic lung disease of prematurity, she was born at 29 weeks’ gestational age and received extracorporeal membrane oxygenation with PLV for severe respiratory syncytial virus-positive bronchiolitis, and respiratory failure at 3 months of age. The PLV was conducted for 24 hours and was performed by instilling perfluorocarbon at a rate of 1 mL/kg of body weight per minute into the lungs through the endotracheal tube, while maintaining continuous mechanical gas ventilation.
In her early years of life she had multiple episodes of bronchiolitis requiring intensive care unit admissions.
A chest radiograph from a recent admission to the emergency department for an asthma exacerbation showed hyperinflation, but no other gross abnormality (figure 1).
Figure 1.
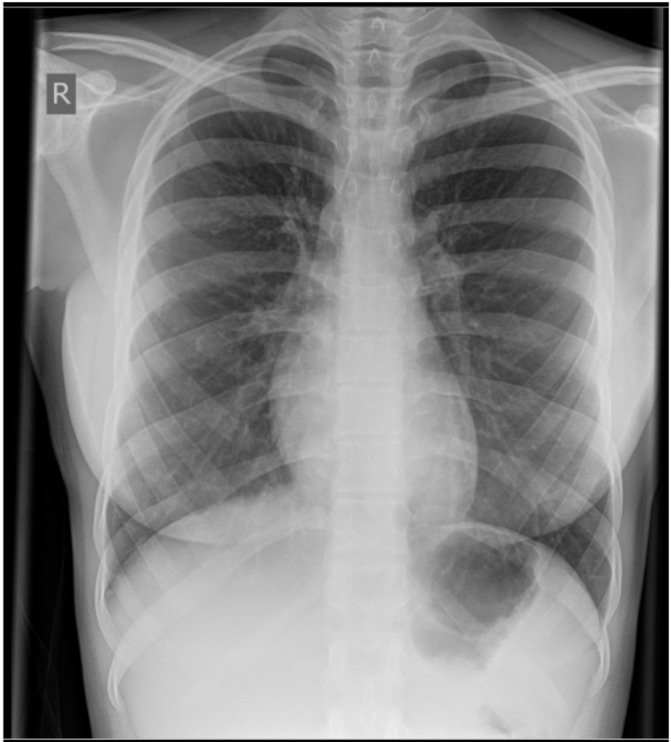
A chest radiograph of the patient at 15 years of age, showing no obvious abnormality.
Recent spirometry showed a moderate obstructive pattern with FEV1 41% predicted.
A high-resolution CT (HRCT) chest scan was performed in view of her ongoing respiratory symptoms and neonatal history.
The HRCT scan demonstrated hyperinflated lungs and emphysematous change consistent with the effects of chronic lung disease of prematurity. Multiple nodules of opacification were seen (figure 2). These were principally pleurally based on the posterolateral aspect of the right lung, over the right diaphragm, along the larger bronchi bilaterally and alongside the aorta. The largest nodule measured 7 mm in diameter and roughly 690 Hounsfield units. This nodule was seen on the right side in a peribronchial location at the hilum (figure 3). In contrast, the lung parenchyma showed no calcified nodules. There was no convincing evidence of bronchiectasis or fibrosis.
Figure 2.
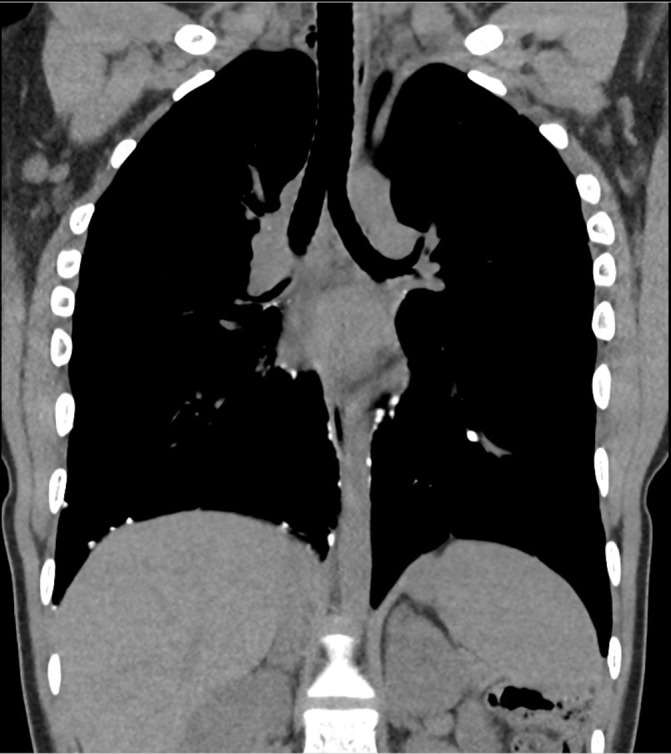
A high-resolution CT (HRCT) image showing multiple nodules of opacification in the lung.
Figure 3.
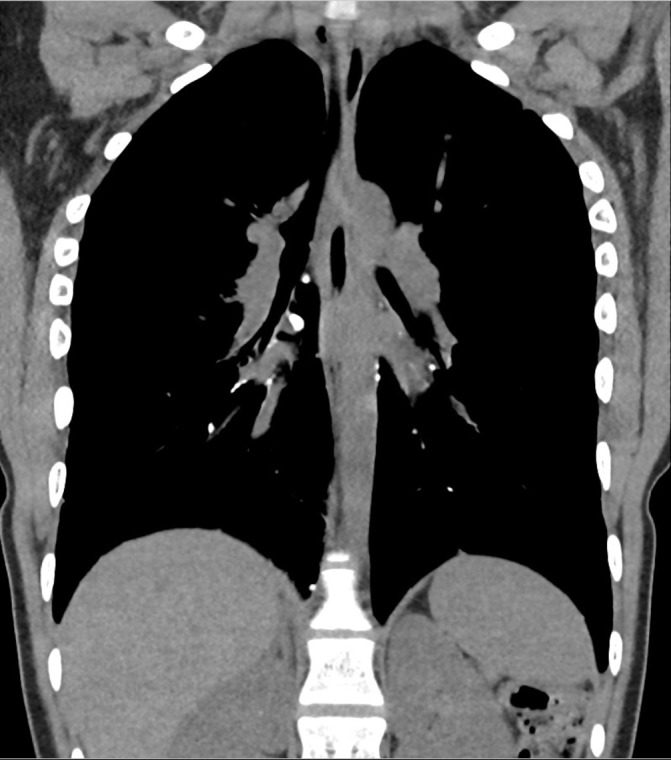
A high-resolution CT (HRCT) image showing the largest nodule of opacification, measuring 7 mm in diameter.
Alpha-1 antitrypsin level was within normal limits at 1.5 g/L and no alpha-1 antitrypsin deficiency allele was identified.
Outcome and follow-up
As the patient has intermittently raised exhaled nitric oxide and clear evidence of bronchial hyper-reactivity and reversibility, she is currently treated with inhaled corticosteroid and salbutamol for probable asthma.
Discussion
When a bromide ion is attached to perfluorocarbon, it appears radiopaque.3 The resultant C8F17Br molecule is insoluble in fat and water.12 Given that perfluorocarbons have high volatility and low surface tension, their elimination from the lungs is through evaporation.12 13 The Hounsfield units of the nodules on our patient’s CT scan are in keeping with the known Hounsfield units value of perfluorocarbon.14
The application of perfluorocarbons is diverse given its physical properties. It is used in vitreoretinal surgery in procedures such as fixing a detached retina, and there are case reports of persistent ocular perfluorocarbon after vitreoretinal surgery.15 16
Two cases have been reported in the literature of perfluorocarbon detection long after liquid ventilation; one was seen 9 years after therapy on chest radiograph,17 the other 12 years after therapy seen on both chest radiograph and CT.18 Both cases were picked up incidentally. Perfluorocarbon has been seen to extravasate into extraparenchymal locations in previous reports, including into the mediastinum, retroperitoneum and lymph nodes.17–20
Given that these patients received liquid ventilation under the pressure of the mechanical gas ventilator, it may follow that rupture of the alveoli through barotrauma results in leakage of the perfluorocarbon into unaerated spaces where it cannot evaporate. Inertness and insolubility in lipid and water may then account for its long-term persistence.
Indeed in our patient, the nodules were not seen within the lung parenchyma but rather within the pleural spaces and within the distribution of the lymphatic uptake of the lung.
During the patient’s neonatal period there was no evidence of air leaks in the form of pneumothorax or pulmonary interstitial emphysema, but both of these would be important risk factors to consider as they would increase the likelihood of pleural extravasation of perfluorocarbon.
Learning points.
Liquid ventilation therapy may be associated with persistent radiopacities on imaging.
Perfluorocarbon residue can persist for 15 years after the initial ventilation therapy.
In patient populations who have received liquid ventilation therapy, it is important to consider persistent perfluorocarbon residue as an explanation for radiopaque abnormalities on imaging.
Footnotes
Contributors: Gathering of information, analysis and researching of the report have been done by ST. MB has critically revised and amended the report.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hamilton MC, Peek GJ, Dux AE, et al. . Partial liquid ventilation. Pediatr Radiol 2005;35:1152–6. 10.1007/s00247-005-1548-x [DOI] [PubMed] [Google Scholar]
- 2.Leach CL, Greenspan JS, Rubenstein SD, et al. . Partial liquid ventilation with perflubron in premature infants with severe respiratory distress syndrome. The LiquiVent Study Group. N Engl J Med 1996;335:761–7. 10.1056/NEJM199609123351101 [DOI] [PubMed] [Google Scholar]
- 3.Riess JG. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol 2005;33:47–63. 10.1081/BIO-200046659 [DOI] [PubMed] [Google Scholar]
- 4.Cox CA, Wolfson MR, Shaffer TH. Liquid ventilation: a comprehensive overview. Neonatal Netw 1996;15:31–43. [PubMed] [Google Scholar]
- 5.Hirschl RB, Parent A, Tooley R, et al. . Liquid ventilation improves pulmonary function, gas exchange, and lung injury in a model of respiratory failure. Ann Surg 1995;221:79–88. 10.1097/00000658-199501000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernan LJ, Fuhrman BP, Kaiser RE, et al. . Perfluorocarbon-associated gas exchange in normal and acid-injured large sheep. Crit Care Med 1996;24:475–81. 10.1097/00003246-199603000-00018 [DOI] [PubMed] [Google Scholar]
- 7.Tütüncü AS, Faithfull NS, Lachmann B. Intratracheal perfluorocarbon administration combined with mechanical ventilation in experimental respiratory distress syndrome: dose-dependent improvement of gas exchange. Crit Care Med 1993;21:962–9. [DOI] [PubMed] [Google Scholar]
- 8.Hirschl RB, Croce M, Gore D, et al. . Prospective, randomized, controlled pilot study of partial liquid ventilation in adult acute respiratory distress syndrome. Am J Respir Crit Care Med 2002;165:781–7. 10.1164/ajrccm.165.6.2003052 [DOI] [PubMed] [Google Scholar]
- 9.Kacmarek RM, Wiedemann HP, Lavin PT, et al. . Partial liquid ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;173:882–9. 10.1164/rccm.200508-1196OC [DOI] [PubMed] [Google Scholar]
- 10.Galvin IM, Steel A, Pinto R, et al. . Partial liquid ventilation for preventing death and morbidity in adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2013:CD003707 10.1002/14651858.CD003707.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushal A, McDonnell CG, Davies MW. Partial liquid ventilation for the prevention of mortality and morbidity in paediatric acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2013:CD003845 10.1002/14651858.CD003845.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaffer TH, Wolfson MR, Clark LC. Liquid ventilation: state of the art review. Pediatr Pulmonol 1992;14:102–9. [DOI] [PubMed] [Google Scholar]
- 13.Shaffer TH, Foust R, Wolfson MR, et al. . Analysis of perfluorochemical elimination from the respiratory system. J Appl Physiol 1997;83:1033–40. 10.1152/jappl.1997.83.3.1033 [DOI] [PubMed] [Google Scholar]
- 14.Hirschl RB, Overbeck MC, Parent A, et al. . Liquid ventilation provides uniform distribution of perfluorocarbon in the setting of respiratory failure. Surgery 1994;116:159–67. [PubMed] [Google Scholar]
- 15.Yu Q, Liu K, Su L, et al. . Perfluorocarbon liquid: its application in vitreoretinal surgery and related ocular inflammation. Biomed Res Int 2014;2014:1–6. 10.1155/2014/250323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirshahi A, Ghasemi F, Zarei M, et al. . Removal of subfoveal perfluorocarbon liquid: Report of 3 cases. J Curr Ophthalmol 2017;29:324–8. 10.1016/j.joco.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagerty RD, Phelan MP, Morrison SC, et al. . Radiographic detection of perflubron fluoromediastinum and fluororetroperitoneum 9 years after partial liquid ventilation. Emerg Radiol 2008;15:71–5. 10.1007/s10140-007-0673-2 [DOI] [PubMed] [Google Scholar]
- 18.Servaes S, Epelman M. Perflubron residua: 12 years following therapy. Pediatr Radiol 2009;39:393–5. 10.1007/s00247-008-1139-8 [DOI] [PubMed] [Google Scholar]
- 19.Meaney JF, Kazerooni EA, Garver KA, et al. . Acute respiratory distress syndrome: CT findings during partial liquid ventilation. Radiology 1997;202:570–3. 10.1148/radiology.202.2.9015092 [DOI] [PubMed] [Google Scholar]
- 20.Jamadar DA, Kazerooni EA, Hirschl RB. Pneumomediastinum: elucidation of the anatomic pathway by liquid ventilation. J Comput Assist Tomogr 1996;20:309–11. 10.1097/00004728-199603000-00027 [DOI] [PubMed] [Google Scholar]


