Figure 4.
M. leprae Alters Nerve Ultrastructure
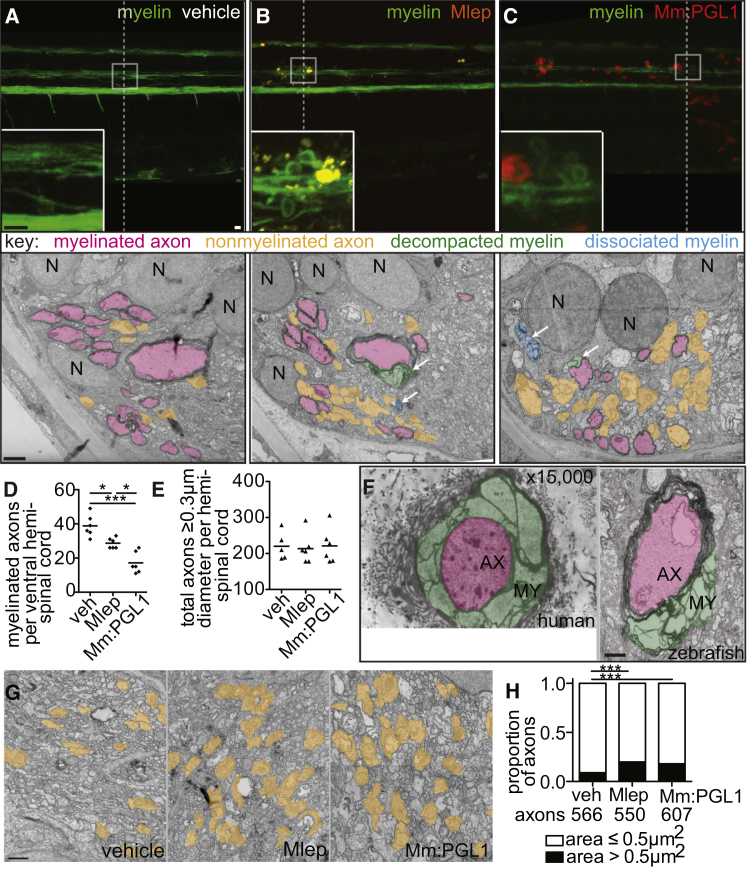
(A–C) Representative confocal images of the spinal cord injection site (upper; scale bar, 10 μm) in mbp larvae at 2 dpi (5 dpf). Insets show magnifications of boxed regions; dashed lines indicate approximate location of the TEM section, shown below with 1 μm scale bars. Highlights indicate myelinated axons (pink), nonmyelinated axons (orange), decompacted myelin (green highlight and arrows), and myelin dissociated from axons (blue highlight and arrows). N, neuronal cell body. Apparent yellow in (B) is due to colocalization of red M. leprae and green myelin and bleed-through of the red PKH into the green channel.
(D and E) Mean number of myelinated axons (D) and total axons (E) per hemi-spinal cord section in larvae injected with PBS vehicle (veh), M. leprae, or M. marinum:PGL-1. (Two hemi-spinal cords scored per larvae; three larvae per group; one-way ANOVA with Bonferonni’s post-test; ∗p < 0.05; ∗∗∗p < 0.001.)
(F) Myelin decompaction in a radial nerve biopsy from a leprosy patient (left) (Job 1973, republished with permission), compared to similar alterations in the myelin of a M. leprae-infected larva (right). MY, myelin; AX, axon; highlights indicate myelinated axons (pink) and decompacted myelin (green); scale bar, 1 μm.
(G) TEMs of larvae obtained like in (A), through matched anatomical regions. Nonmyelinated axons with diameter ≥ 0.5 μm2 are highlighted in orange; scale bar, 1 μm.
(H) Proportion of nonmyelinated axons with area >0.5 or ≤0.5 μm2 from larvae obtained like in (A) (∗∗∗p < 0.001; Fisher’s exact test).
See also Figure S3.