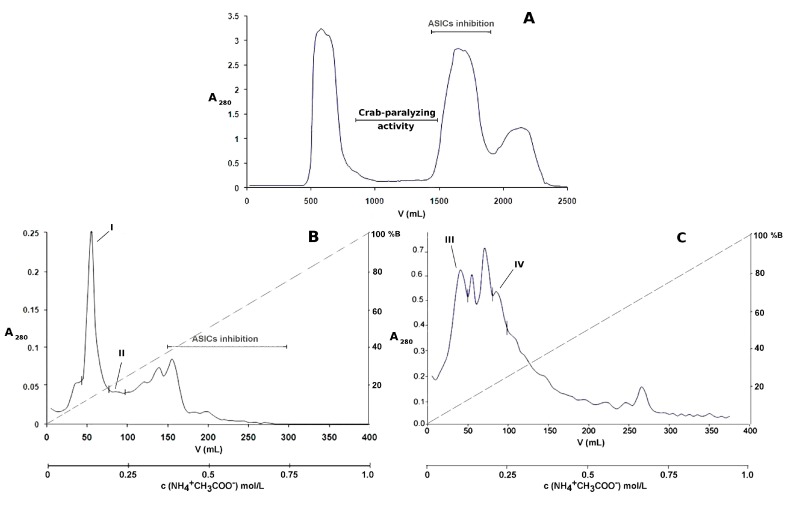
Figure 1.
(A) Gel filtration profile of P. crucifer aqueous extract. The soluble material contained in 5 grams of whole-body homogenate (350 mg/90 mL) was fractionated on Sephadex G-50 (5 × 93 cm) at 2 mL/min using 0.1 mol/L ammonium acetate. Fractions of 20 mL each were collected; those within the elution volumes of 820 mL to 1460 mL were paralyzing to all of the crabs, and were pooled; (B) Cation-exchange chromatographic profile of the crab-paralyzing pool of chromatographic fractions from Sephadex G-50, in Fractogel EMD SO3− 650 M (1.8 × 5 cm); (C) Anion-exchange chromatographic profile of the non-retained fraction from the cation exchanger, in Fractogel EMD DEAE 650 M (1.8 × 5 cm). Both separations (B,C) were done at a flow rate of 1 mL/min using a 400-mL gradient, from 0.01 mol/L to 1 mol/L ammonium acetate. Eighty fractions of 5 mL each were collected in every chromatographic separation. Dashed lines in the ion-exchange chromatographic profiles represent the gradient of ammonium acetate. Fractions exhibiting toxicity to crabs were named I, II, III, and IV. The pools of fractions that inhibited acid-sensing ion channels are shown in both gel-filtration and cation-exchange chromatographic profiles, according to previous results with the same P. crucifer homogenate, using identical conditions [6]. PhcrTx1, an acid-sensing ion channel toxin from P. crucifer [6], eluted inhibiting pools of chromatographic fractions in the ASICs, as shown in (A,B). As shown, the crab-paralyzing zone and the ASICs inhibition zone barely overlapped in the gel filtration profile (A); and completely separated from each other in the cation-exchange profile (B). PhcrTx1 is not present among the crab-paralyzing chromatographic fractions isolated from the ion-exchange chromatographic separations.