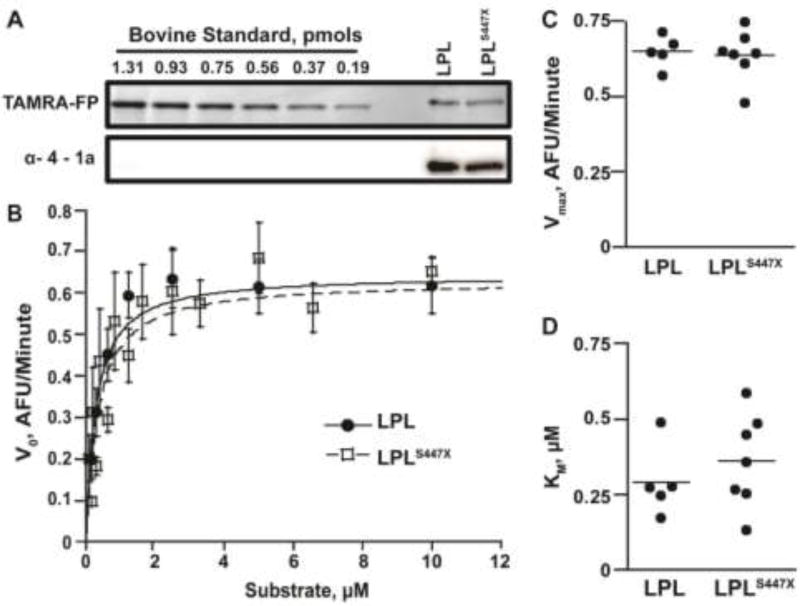
Figure 1.
LPL and LPLS447X are equally active on the lipase substrate DGGR. A) An activity based probe was used to quantify the amount of active human LPL and LPLS447X using bovine LPL as a standard. The equal concentration of LPL and LPLS447X is verified by Western blot using an antibody more sensitive to human LPL. B) A Michaelis–Menten curves showing that LPL (filled circles) and LPLS447X (empty squares) have identical activity when hydrolyzing a synthetic substrate. C) Graph of Vmax and Km values from multiple experiments for both LPL variants. A two-tailed student’s t-test showed no significant difference in Vmax (p > 0.05) and Km (p > 0.05) for LPL vs. LPLS447X.