Summary
Among patients with suspected drug resistant tuberculosis, treatment with a later generation fluoroquinolone versus earlier generation or no fluoroquinolone significantly reduced mortality risk after adjusting for risk factors typically associated with poor outcomes.
Keywords: drug resistance, tuberculosis, mortality, fluoroquinolones
Abstract
Background
Previous retrospective and in vitro studies suggest that use of later-generation fluoroquinolones may reduce mortality risk and improve treatment outcomes for drug-resistant tuberculosis (TB) patients, including individuals resistant to a fluoroquinolone. Meta-analysis results are mixed and few studies have examined this relationship prospectively.
Methods
As part of a comparative diagnostic study, we conducted a prospective cohort study with 834 Mycobacterium tuberculosis–infected patients from selected hospitals and clinics with high prevalence of drug-resistant TB in India, Moldova, and South Africa. We used Cox proportional hazards regression models to assess the association between later-generation fluoroquinolone (moxifloxacin or levofloxacin) use and patient mortality, adjusting for risk factors typically associated with poor treatment outcomes.
Results
After adjusting for phenotypic resistance profile, low body mass index (<18.5 kg/m2), human immunodeficiency virus status, and study site, participants treated with a later-generation fluoroquinolone had half the risk of mortality compared with participants either not treated with any fluoroquinolone or treated only with an earlier-generation fluoroquinolone (adjusted hazard ratio, 0.46 [95% confidence interval, .26–.80]) during follow-up.
Conclusions
Use of later-generation fluoroquinolones significantly reduced patient mortality risk in our cohort, suggesting that removal of a later-generation fluoroquinolone from a treatment regimen because of demonstrated resistance to an earlier-generation fluoroquinolone might increase mortality risk. Further studies should evaluate the effectiveness of later-generation fluoroquinolones among patients with and without resistance to early-generation fluoroquinolones.
Clinical Trials Registration
Although incidence rates of tuberculosis (TB) are decreasing globally, TB remains the leading cause of infectious disease death worldwide [1]. In 2015, the World Health Organization (WHO) estimated that there were 10.4 million new TB cases and 1.4 million TB-related deaths [2]. Drug-resistant disease accounts for a disproportionate number of these deaths [3, 4]. Mortality rates among drug-susceptible patients are typically <10%, while rates among patients with extensively drug-resistant (XDR) TB (ie, bacillary resistance to isoniazid and rifampicin, any fluoroquinolone, and any second-line injectable [kanamycin, capreomycin, or amikacin]) have been reported to be as high as 75%; in the seminal KwaZulu-Natal XDR-TB outbreak, mortality reached 98% [1, 5–7].
Poor treatment outcomes have been associated with prior treatment for TB, history of smoking, diabetes, human immunodeficiency virus (HIV) infection, low body weight, and sputum smear positivity [4, 8–11]. In addition, among drug-resistant TB patients, increased risk of mortality has been attributed to the limited number of potentially effective drugs for patients with resistant strains of Mycobacterium tuberculosis (Mtb) [12]. The repurposing of fluoroquinolones typically reserved for treating drug-resistant TB for use in newer shorter-course treatments for drug-susceptible TB adds further complexity to treatment options for drug-resistant TB [13, 14].
In vitro analysis has demonstrated that resistance to early-generation fluoroquinolones (ciprofloxacin or ofloxacin) does not always predict cross-resistance to later-generation fluoroquinolones (levofloxacin, moxifloxacin, or gatifloxacin) [15, 16]. Multiple retrospective studies suggest that use of later-generation fluoroquinolones, even among strains that are resistant to an early-generation fluoroquinolone, improve treatment outcomes and reduce mortality risk [4, 8, 17–19]. Additionally, murine models have demonstrated that later-generation fluoroquinolones can be used to successfully treat fluoroquinolone-resistant strains of Mtb, specifically those that harbor mutations associated with low minimum inhibitory concentrations (MICs) to early-generation fluoroquinolones [20, 21]. Although the WHO currently recommends treatment of drug-resistant TB with later-generation fluoroquinolones [22, 23], few studies have prospectively examined the association between later-generation fluoroquinolone use and risk of mortality [5, 12, 24].
In this prospective cohort study, we evaluated the impact of later-generation fluoroquinolone vs early-generation or no fluoroquinolone use on mortality risk among patients with diverse drug resistance profiles, taking into account risk factors that have been previously associated with poor TB patient outcomes.
METHODS
Data for this analysis were collected as part of a large multisite prospective cohort study (ClinicalTrials.gov registration number: NCT02170441) conducted by the Global Consortium for Drug-Resistant TB Diagnostics, which was designed to compare multiple rapid diagnostic assays for the detection of drug-resistant TB.
Institutional review board approval for this study was received from the University of California, San Diego and from participating institutions at their respective study sites. Participation did not alter the standard of care for participants.
Detailed descriptions of the study protocols have been published previously [25, 26]. In brief, between 2012 and 2013, patients presenting with suspected drug-resistant TB at participating clinic study sites in India, Moldova, and South Africa were screened, consented, and invited to participate. Inclusion criteria for the study were evidence of active TB disease [1], either with acid-fast bacilli (AFB) smear positive, GeneXpert positive, or a high clinical suspicion of TB, and suspected drug resistance [2], defined as having previously received >1 month of treatment for a prior TB episode, failing TB treatment with positive sputum smear or culture after ≥3 months of a standard TB treatment, having close contact with a known drug-resistant TB case, being diagnosed with multidrug-resistant (MDR) TB (defined as resistance to both isoniazid and rifampicin) within the last 30 days, or being previously diagnosed with MDR-TB and failing TB treatment with positive sputum smear or culture after ≥3 months of a standard MDR-TB treatment regimen.
Specimen Collection and Laboratory Methods
Pooled sputum specimens used for analysis were comprised of sputum collected at enrollment and again on the following morning upon waking. Susceptibility testing was performed on all culture-positive sputum specimens using the reference phenotypic assay, MGIT960 (BD Biosciences, Sparks, Maryland). Susceptibility to isoniazid, rifampicin, moxifloxacin, ofloxacin, kanamycin, capreomycin, and amikacin were assessed using WHO critical concentrations of 0.1, 1.0, 0.25, 2.0, 2.5, 2.5, and 1.0 μg/mL, respectively, according to the manufacturer’s instructions. Critical concentrations for fluoroquinolones were based on the 2008 WHO guidelines on drug susceptibility testing (DST) of second-line antituberculosis drugs [27]. In 2012, after the initiation of our study, the StopTB Partnership proposed splitting the critical concentration for moxifloxacin into 2 categories (0.5 and 2.0 μg/mL), but this recommendation has not been adopted by the WHO [28].
Data Collection and Variable Construction
Participants were interviewed upon enrollment and again approximately 52 weeks after enrollment. Demographic data, comorbidity data, and other risk factor data were collected at enrollment. Dichotomous variables including HIV status, smoking history, previous TB treatment, and diabetic status were defined as participants with the risk factor being assessed vs participants without risk factor or unknown risk factor status. Drugs used during the initial phase of treatment for the current TB episode were extracted from the medical records at enrollment and approximately 30 days postenrollment. Cases treated with a later-generation fluoroquinolone were defined as individuals who had a record of treatment with either moxifloxacin or levofloxacin (there were no records of treatment with gatifloxacin). The comparison group consisted of individuals not treated with a later-generation fluoroquinolone, which included those treated with an earlier-generation fluoroquinolone (ofloxacin or sparfloxacin) or no fluoroquinolone. Among those with a record of death, follow-up time was calculated from enrollment date to date of death, if recorded. In instances where the date of death included only the month and year, the date of death was calculated to the 15th of the month. Participants classified as deceased without a recorded date of death were excluded from the proportional hazards regression analysis but were included in the overall mortality statistics. Follow-up time for participants not classified as deceased was calculated from enrollment to last date of contact, either the 30-day medical record review or the 52-week follow-up interview. If no data were collected for an individual beyond enrollment, that individual was excluded from all analyses. Drug resistance profile was categorized as follows: susceptible, monoresistant (resistant to either rifampicin or isoniazid), MDR (resistant to rifampicin and isoniazid), pre–XDR (resistant to either a second-line injectable drug or a fluoroquinolone but not both), or XDR (resistant to rifampicin, isoniazid, a second-line injectable drug, and a fluoroquinolone).
Statistical Analysis
Patient demographics and clinical data were described using median and interquartile range (IQR) for continuous variables and frequency and proportion for categorical variables. Statistical significance was set at .05 for all analyses. Kaplan-Meier curves were used to compare mortality by drug resistance category. Cox proportional hazards models were used to determine hazard ratios (HRs) and 95% confidence intervals (CIs) for all survival analyses. The association between each risk factor and mortality was adjusted for resistance profile in the bivariate analysis initially. All covariates with a P < .25 in the bivariate analysis and covariates frequently associated with TB mortality were included in the preliminary multivariable model. The final multivariable model included variables significant at the P = .05 level, HIV, and study site. Proportional hazard assumptions were visually assessed using log-log plots and Schoenfeld residuals. All analyses were conducted in Stata software version 13.1 (StataCorp, College Station, Texas).
RESULTS
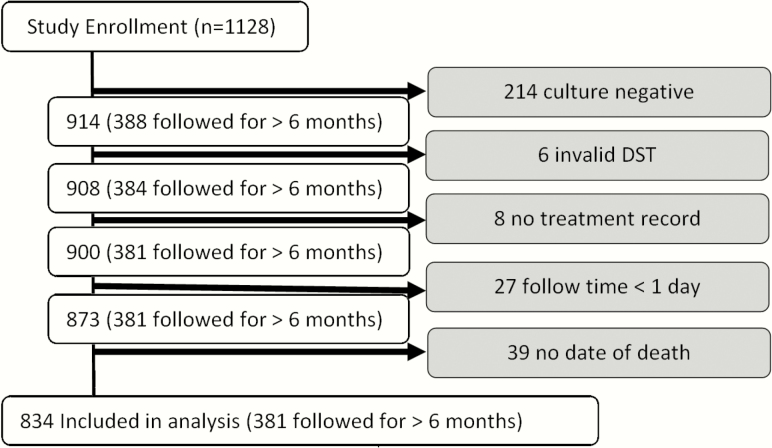
Of the total 1128 participants enrolled in the parent study, 214 produced culture-negative specimens, 6 yielded invalid DST results, 8 had no record of drug treatment, and 27 lacked any follow-up data. Of the remaining 873 (77%) participants, 101 had a record of death; however, 39 of those participants had no date of death recorded and were excluded from the Cox proportional hazards analysis, resulting in a study sample of 834 (74%) (Figure 1). Participants were followed for a mean of 190 days (median, 91 [IQR, 31–365] days). Forty-two percent of participants completed their final “52 week” visit (range, 32–72 weeks postenrollment).
Figure 1.
Selection of participants for analysis. Abbreviation: DST, drug susceptibility testing.
Of the 834 participants included in this analysis, 65% were male. The median age of the population was 33, and 75% reported being previously treated for a prior episode of TB. Thirty-six percent of participants reported smoking at baseline, 11% were HIV positive, 5% were diabetic, and 54% had a body mass index (BMI) <18.5 kg/m2.
Overall, 34% (282) were susceptible to all tested drugs, 6% (52) were monoresistant, 18% (153) were MDR, 34% (290) were pre-XDR, and 7% (57) were XDR (Table 1). A total of 279 participants were ofloxacin resistant; of those, 272 (97%) were cross-resistant to moxifloxacin. Of the 379 study participants who had a record of treatment with a later-generation fluoroquinolone (moxifloxacin or levofloxacin), nearly half (48%) were phenotypically resistant to ofloxacin. No patient had a record of treatment with gatifloxacin.
Table 1.
Clinical Characteristics at Baseline and Bivariate Hazard Ratios Adjusted for Resistance Category
| Variable | Total (N = 834) | Survived (n = 772) | Died (n = 62) | Adjusted HR (N = 834) | P Value | (95% CI) |
|---|---|---|---|---|---|---|
| Resistance category | ||||||
| Susceptible (reference) | 282 (34) | 272 (35) | 10 (16) | … | … | … |
| Monoresistance to RIF/INH | 52 (6) | 49 (6) | 3 (5) | … | … | … |
| MDR | 153 (18) | 143 (19) | 10 (16) | … | … | … |
| Second-line resistance | 290 (34) | 262 (34) | 28 (45) | … | … | … |
| XDR | 57 (7) | 46 (6) | 11 (18) | … | … | … |
| Site | ||||||
| India (reference) | 445 (53) | 409 (53) | 36 (58) | 1.00 | … | … |
| Moldova | 225 (27) | 208 (27) | 17 (27) | 1.06 | .84 | (.59–1.92) |
| South Africa | 164 (20) | 155 (20) | 9 (15) | 2.32 | .08 | (.90–5.99) |
| HIV positive (vs negative or unknown) | 91 (11) | 84 (11) | 7 (11) | 3.11 | .01 | (1.31–7.37) |
| Smear category | ||||||
| Negative (reference) | 134 (16) | 128 (17) | 6 (10) | 1.00 | … | … |
| Rare | 70 (8) | 64 (8) | 6 (10) | 2.22 | .17 | (.71–6.96) |
| Few | 164 (20) | 158 (20) | 6 (10) | 0.89 | .85 | (.29–2.79) |
| Many | 169 (20) | 150 (19) | 19 (31) | 2.42 | .06 | (.96–6.11) |
| Too numerous to count | 297 (36) | 272 (35) | 25 (40) | 1.62 | .30 | (.65–4.01) |
| Sputum smear positive | 700 (84) | 644 (83) | 56 (90) | 1.73 | .21 | (.74–4.05) |
| History of smoking (vs no history or unknown) | 302 (36) | 278 (36) | 24 (39) | 1.43 | .19 | (.84–2.46) |
| Previous treatment | 624 (75) | 582 (75) | 47 (75) | 1.05 | .88 | (.58–1.88) |
| Low BMI (<18.5 kg/m2) | 448 (54) | 406 (53) | 42 (68) | 1.96 | .01 | (1.15–3.35) |
| Diabetes | 44 (5) | 40 (5) | 4 (6) | 0.95 | .92 | (.34–2.62) |
| Male sex | 542 (65) | 500 (65) | 42 (68) | 1.44 | .19 | (.84–2.47) |
| Use of a later-generation FQ | 379 (45) | 352 (46) | 27 (44) | 0.48 | .01 | (.28–.83) |
| Age continuous, median (Q1, Q3) | 33 (24, 45) | 33 (24, 46) | 36 (23, 44) | 1.01 | .43 | (.99–1.02) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; CI, confidence interval; FQ, fluoroquinolone; HIV, human immunodeficiency virus; HR, hazard ratio; INH, isoniazid; MDR, multidrug resistant; RIF, rifampin; XDR, extensively drug resistant.Values in bold indicate significant differences at the P > .05 level.
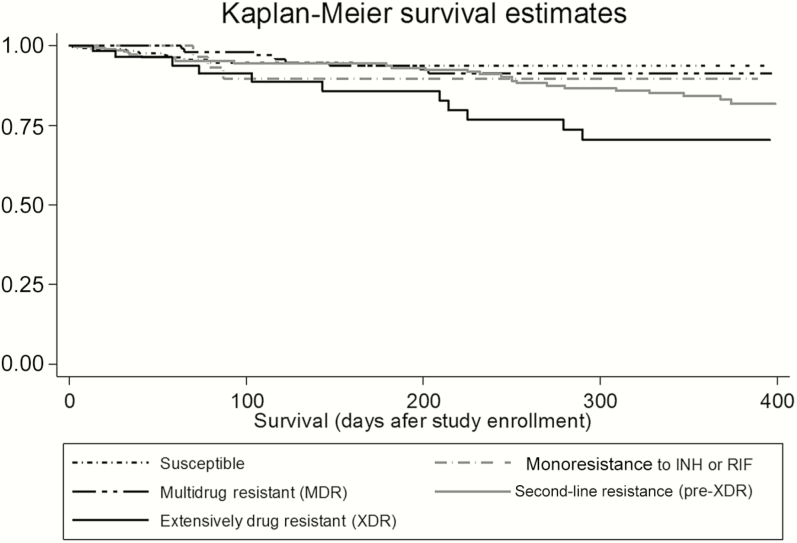
If participants without a date of death were included in mortality calculations (n = 873), overall mortality was 11.6% and among participants with XDR-TB mortality was 37.8%. In the study sample used for proportional hazards analysis (N = 834), overall mortality was 7.4%. Mortality rates among participants with susceptible TB, monoresistant TB, and MDR-TB were similar (Figure 2). Participants with XDR-TB had a significantly higher mortality rate than participants with MDR-TB (19.3% vs 6.5%, P = .02).
Figure 2.
Kaplan-Meier survival analysis estimates of survival stratified by drug resistance category (N = 834). Abbreviations: INH, isoniazid; MDR, multidrug resistant; RIF, rifampin; XDR, extensively drug resistant.
The preliminary main effects model included variables associated with mortality or poor outcomes in previous studies (Table 2). All variables that were not significant in the preliminary model (history of smoking, sputum smear positive, previous treatment, diabetes, age, and sex) were excluded in the final model with the exception of HIV status and study site. Removing these variables together did not significantly reduce the model fit. In the final model, the risk of mortality increased as level of resistance increased, culminating in XDR-TB participants having 9 times greater risk of mortality compared to drug-susceptible participants (adjusted HR, 9.01 [95% CI, 3.11–26.1]) after adjusting for low BMI, HIV status, treatment with later-generation fluoroquinolones, and study site.
Table 2.
Preliminary Main Effects Multivariable Regression Model and Final Model
| Preliminary Model | Final Model | |||
|---|---|---|---|---|
| Factor | Hazard Ratio | (95% CI) | Hazard Ratio | (95% CI) |
| Later-generation FQs | 0.45 | (.26–.80) | 0.46 | (.26–.80) |
| Resistance category | ||||
| Susceptible (reference) | 1.00 | … | 1.00 | … |
| Monoresistance to RIF or INH | 1.57 | (.43–5.78) | 1.76 | (.48–6.45) |
| MDR | 3.27 | (1.15–9.30) | 3.23 | (1.11–9.41) |
| Pre-XDR | 5.42 | (2.12–13.9) | 5.31 | (2.02–12.1) |
| XDR | 10.2 | (3.52–29.8) | 9.01 | (3.11–26.1) |
| Study site | ||||
| India (reference) | 1.00 | … | 1.00 | … |
| Moldova | 0.89 | (.31–2.51) | 1.48 | (.79–2.77) |
| South Africa | 1.57 | (.45–5.53) | 1.68 | (.52–5.47) |
| Smear status | 1.91 | (.79–4.64) | … | … |
| BMI <18.5 kg/m2 | 2.21 | (1.25–3.91) | 2.10 | (1.20–3.65) |
| HIV positive (vs negative or unknown) | 2.41 | (.85–6.79) | 2.46 | (.83–7.32) |
| Smoker | 1.23 | (.58–2.59) | … | … |
| Previous TB treatment | 0.66 | (.26–1.67) | … | … |
| Diabetes | 0.87 | (.28–2.70) | … | … |
| Age | 1.01 | (.99–1.03) | … | … |
| Male (vs female) | 1.55 | (.84–2.85) | … | … |
Abbreviations: BMI, body mass index; CI, confidence interval; FQ, fluoroquinolone; HIV, human immunodeficiency virus; INH, isoniazid; MDR, multidrug resistant; RIF, rifampin; TB, tuberculosis; XDR, extensively drug resistant.Values in bold indicate significant differences at the P > .05 level.
Treatment with later-generation fluoroquinolones was associated with >50% reduced risk of mortality (adjusted HR, 0.46 [95% CI, .26–.80]) compared to treatment with an earlier-generation fluoroquinolone or no treatment with any fluoroquinolone regardless of drug resistance profile, HIV status, and study site. Participants with a BMI of <18.5 kg/m2 had twice the risk of mortality compared to participants with a BMI of ≥18.5 kg/m2 (adjusted HR, 1.96 [95% CI, 1.15–3.35]) after adjusting for drug resistance profile, HIV status, study site, and treatment with a later-generation fluoroquinolone.
Among XDR-TB participants only (n = 57), use of later-generation fluoroquinolones was not significantly associated with a reduced risk of mortality after controlling for study site, low BMI, and HIV status. Among non-XDR patients however, use of later-generation fluoroquinolones was significantly associated with a reduced risk of mortality (adjusted HR, 0.54 [95% CI, .29–.98]) after adjusting for study site, low BMI, and HIV status. Among fluoroquinolone (ofloxacin and/or moxifloxacin)–resistant participants only, the use of moxifloxacin did not reduce mortality risk (adjusted HR, 1.62 [95% CI, .77–3.40]) after adjusting for study site, low BMI, and HIV status. In addition, the association between the use of levofloxacin and reduced mortality risk was not significant (adjusted HR, 0.49 [95% CI, .22–1.07]) after adjusting for study site, low BMI, and HIV status.
DISCUSSION
This prospective study assessed the impact of later-generation fluoroquinolone use on mortality among a cohort of participants with diverse drug resistance profiles. In our study population, participants with a record of later-generation fluoroquinolone use had half the risk of mortality compared with participants who had a record of earlier-generation fluoroquinolone use or no record of fluoroquinolone use after adjusting for drug resistance profile, HIV status, BMI, and study site.
A subanalysis of patients harboring phenotypic early-generation fluoroquinolone resistance revealed that the use of later-generation fluoroquinolones appeared to be protective, although the association was not statistically significant. This may be due to the nearly complete (97%) cross-resistance observed between early- and later-generation fluoroquinolones in our study population. Results may have been more conclusive if there had been less cross-resistance, as demonstrated previously by Jo et al [29].
Our findings confirm what previous retrospective studies have identified: a positive association between later-generation fluoroquinolone use and treatment success [18, 19]. Comparison to results of recent meta-analyses are mixed. Both Jacobson et al and Isaakidis et al demonstrated that the use of fluoroquinolones among XDR-TB and HIV/MDR-TB was associated with improved treatment outcomes and treatment success [8, 17]. In contrast, a large meta-analysis conducted by Falzon et al using patient level resistance and treatment data from a diverse cohort with multiple resistance profiles failed to show any association between treatment with fluoroquinolones and successful outcomes among fluoroquinolone-resistant participants [30]. Lack of consistent results may be due to the infrequent use of later-generation fluoroquinolones in treatment regimens, failure to differentiate between early- and later-generation fluoroquinolone use during analysis, or reliance on the results of a single, early-generation drug susceptibility test (ie, ofloxacin) for both early- and later-generation fluoroquinolone resistance determination.
Few prospective studies have assessed fluoroquinolone use, specifically, later-generation fluoroquinolone use among drug-resistant patients, and to our knowledge, our study is one of the first prospective studies to identify an association between later-generation fluoroquinolone use and a reduction in mortality risk. The Preserving Effective Tuberculosis Treatment Study (PETTS), a multicountry prospective cohort study, compared the total number of effective drugs and their association with culture conversion at specified time points, and identified an association between use of any fluoroquinolone and an increased treatment success [12, 31]. In contrast to the current study, the PETTS did not account for typical risk factors associated with poor outcomes or level of drug resistance in their models, and associations were all reported as pairwise comparisons. In a single-country prospective study, Pietersen et al also did not find an association between fluoroquinolone use (either ofloxacin or moxifloxacin) and mortality; however, the study sample included only XDR-TB patients in contrast to the current study which included suspect drug-resistant TB patients with varying levels of drug resistance [5].
The complexity of assessing effectiveness of an entire class of fluoroquinolones in the context of multiple drug regimens cannot be underestimated. Clinical studies designed to assess susceptibility to and effectiveness of individual fluoroquinolones in the context of a multiple drug regimens, including studies investigating the complex relationships between treatment regimens, clinical data, and molecular test results (eg, sequencing data), are needed to confirm these findings.
Resistance Profiles
Notably, and as demonstrated previously, drug resistance profile appeared to be the most significant predictor of mortality; patients with drug-susceptible TB had the lowest risk of mortality and patients with resistance to an increasing number of drugs had an increased risk of mortality, with XDR-TB patients having the highest risk of mortality even after adjusting for risk factors typically associated with mortality [10, 30, 32].
Just over 40% of our study cohort completed their final 52-week follow-up visit. As the Kaplan-Meier plot demonstrates, crude mortality rates in our cohort were not significantly different between participants with drug-susceptible, monoresistant, and MDR-TB, with mortality risk occurring primarily during the first 3 months after enrollment, which is consistent with previous studies [33]. In contrast to participants with MDR-TB, participants with XDR-TB had mortality risks that did not appear to level off after the first 3 months, but instead steadily increased for the duration of the study. This continuing risk of mortality even up to 6 months after diagnosis has been demonstrated previously in patients coinfected with HIV [18].
Although HIV has historically been associated with increased mortality among TB patients, in our study HIV was not significantly associated with an increased risk of mortality in the final model after adjusting for low BMI, resistance profile, and study site [8]. Similar findings have been demonstrated previously, and may be due to increased HIV treatment coverage. Diabetes, history of smoking, AFB smear positivity, and previous treatment were also not significantly associated with mortality in our study.
Patients with low BMI (<18.5 kg/m2) had twice the risk of mortality compared to patients with normal or higher BMI (≥18.5 kg/m2) regardless of TB drug resistance profile and comorbidities. Previous studies have suggested that BMI may be a marker of disease severity or lack of treatment response or a proxy for underlying socioeconomic risk factors [34, 35]. Our study confirms that low BMI is associated with poor TB treatment outcomes and underscores the need to aggressively treat patients presenting with low BMI and also to focus efforts on increasing BMI during treatment [4, 36–38].
Our study was subject to several limitations. Follow-up data collection was limited to 2 time points, reducing our ability to quantify duration of treatment. However, the positive association between reduced mortality risk and patients who had a record of treatment with later-generation fluoroquinolones, regardless of treatment duration, increases our confidence in these findings. Our inability to quantify treatment duration also eliminated possible analysis based on regimen composition or change patterns due to drug toxicity. Additionally, 39 participants classified as deceased did not have a date of death recorded and, therefore, were excluded from the proportional hazards regression analysis, potentially biasing our results toward the null. Although we assessed the impact of both levofloxacin and moxifloxacin (later-generation fluoroquinolones) on clinical outcomes, only moxifloxacin was used to determine phenotypic drug susceptibility. Also, data on fluoroquinolone use during a prior TB episode were not collected; as a result, impact of previous fluoroquinolone exposure could not be assessed. Finally, the proportion of our study cohort with XDR-TB was relatively small, reducing our ability to assess risk factors associated with mortality individually for patients with different drug resistance profiles.
In conclusion, treatment of suspected drug-resistant TB with a later-generation fluoroquinolone appeared to reduce patient mortality risk even after adjusting for resistance profile and risk factors typically associated with poor outcomes. Further studies designed to assess the use of later-generation fluoroquinolones on mortality are needed. In addition, individuals with higher levels of resistance or presenting with low BMI appear to be at significantly increased risk of mortality and should be monitored accordingly.
Notes
Acknowledgments. The authors thank Dr Antonino Catanzaro for his oversight and input on this study. We thank the laboratory and clinical staff at P.D. Hinduja Hospital and Medical Research Center in Mumbai, India; the Institute of Phthisiopneumology in Chisinau, Moldova; researchers at Stellenbosch University and the 6 primary health care facilities and regional hospital in Port Elizabeth, South Africa; and laboratory and data management personnel at the University of California, San Diego, for their work and contribution to the GCDD study. We thank the South Africa MRC Centre for TB Research, the Department of Science and Technology-National Research Foundation Centre of Excellence for Biomedical Tuberculosis Research, Division of Molecular Biology and Human Genetics, and University of Stellenbosch for providing the infrastructure for this study in South Africa.
Disclaimer. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies.
Financial support. This work was supported by the National Institutes of Health (U01-AI082229, P30-AI036214, and R01-AI111435 to T. C. R. and T32-HL098062 to M. S.). This work was supported by the Global Consortium for Drug-Resistant TB Diagnostics (GCDD; http://gcdd.ucsd.edu).
Potential conflicts of interest. T. C. R. and S. B. G. receive salary support from the Foundation for Innovative New Diagnostics (FIND), a nonprofit organization. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 2. World Health Organization. Global tuberculosis report 2016. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 3. Migliori GB, Besozzi G, Girardi E et al. ; SMIRA/TBNET Study Group Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J 2007; 30:623–6. [DOI] [PubMed] [Google Scholar]
- 4. Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One 2009; 4:e6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pietersen E, Ignatius E, Streicher EM et al. . Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet 2014; 383:1230–9. [DOI] [PubMed] [Google Scholar]
- 6. Milanov V, Falzon D, Zamfirova M et al. . Factors associated with treatment success and death in cases with multidrug-resistant tuberculosis in Bulgaria, 2009–2010. Int J Mycobacteriol 2015; 4:131–7. [DOI] [PubMed] [Google Scholar]
- 7. Gandhi NR, Moll A, Sturm AW et al. . Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006; 368:1575–80. [DOI] [PubMed] [Google Scholar]
- 8. Isaakidis P, Casas EC, Das M, Tseretopoulou X, Ntzani EE, Ford N. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. Int J Tuberc Lung Dis 2015; 19:969–78. [DOI] [PubMed] [Google Scholar]
- 9. Gadoev J, Asadov D, Tillashaykhov M et al. . Factors associated with unfavorable treatment outcomes in new and previously treated TB patients in Uzbekistan: a five year countrywide study. PLoS One 2015; 10:e0128907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung-Delgado K, Guillen-Bravo S, Revilla-Montag A, Bernabe-Ortiz A. Mortality among MDR-TB cases: comparison with drug-susceptible tuberculosis and associated factors. PLoS One 2015; 10:e0119332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pai M, Mohan A, Dheda K et al. . Lethal interaction: the colliding epidemics of tobacco and tuberculosis. Expert Rev Anti Infect Ther 2007; 5:385–91. [DOI] [PubMed] [Google Scholar]
- 12. Yuen CM, Kurbatova EV, Tupasi T et al. . Association between regimen composition and treatment response in patients with multidrug-resistant tuberculosis: a prospective cohort study. PLoS Med 2015; 12:e1001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee HW, Lee JK, Kim E, Yim JJ, Lee CH. The effectiveness and safety of fluoroquinolone-containing regimen as a first-line treatment for drug-sensitive pulmonary tuberculosis: a systematic review and meta-analysis. PLoS One 2016; 11:e0159827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ziganshina LE, Titarenko AF, Davies GR. Fluoroquinolones for treating tuberculosis (presumed drug-sensitive). Cochrane Database Syst Rev 2013; 6:CD004795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farhat MR, Mitnick CD, Franke MF et al. . Concordance of Mycobacterium tuberculosis fluoroquinolone resistance testing: implications for treatment. Int J Tuberc Lung Dis 2015; 19:339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGrath M, Gey van Pittius NC, Sirgel FA, Van Helden PD, Warren RM. Moxifloxacin retains antimycobacterial activity in the presence of gyrA mutations. Antimicrob Agents Chemother 2014; 58:2912–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2010; 51:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dheda K, Shean K, Zumla A et al. . Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet 2010; 375:1798–807. [DOI] [PubMed] [Google Scholar]
- 19. Kwak N, Kim HR, Yoo CG, Kim YW, Han SK, Yim JJ. Changes in treatment outcomes of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2015; 19:525–30. [DOI] [PubMed] [Google Scholar]
- 20. Fillion A, Aubry A, Brossier F, Chauffour A, Jarlier V, Veziris N. Impact of fluoroquinolone resistance on bactericidal and sterilizing activity of a moxifloxacin-containing regimen in murine tuberculosis. Antimicrob Agents Chemother 2013; 57:4496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poissy J, Aubry A, Fernandez C et al. . Should moxifloxacin be used for the treatment of extensively drug-resistant tuberculosis? An answer from a murine model. Antimicrob Agents Chemother 2010; 54:4765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. 2014. Available at: http://apps.who.int/iris/bitstream/10665/130918/1/9789241548809_eng.pdf. Accessed 22 September 2016. [PubMed] [Google Scholar]
- 23. World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis—2016 update. Geneva, Switzerland: WHO, 2016. [PubMed] [Google Scholar]
- 24. Balabanova Y, Ignatyeva O, Fiebig L et al. . Survival of patients with multidrug-resistant TB in Eastern Europe: what makes a difference? Thorax 2016; 71:854–61. [DOI] [PubMed] [Google Scholar]
- 25. Hillery N, Groessl EJ, Trollip A et al. . The Global Consortium for Drug-Resistant Tuberculosis Diagnostics (GCDD): design of a multi-site, head-to-head study of three rapid tests to detect extensively drug-resistant tuberculosis. Trials 2014; 15:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Catanzaro A, Rodwell TC, Catanzaro DG et al. . Performance comparison of three rapid tests for the diagnosis of drug-resistant tuberculosis. PLoS One 2015; 10:e0136861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. Molecular line probe assays for rapid screening of patients at risk of multi-drug resistant tuberculosis 2008. Available at: http://www.who.int/tb/features_archive/expert_group_report_june08.pdf. Accessed 16 September 2015.
- 28. Stop TB Partnership. Updated interim critical concentrations for first-line and second-line DST Available at: http://www.stoptb.org/wg/gli/assets/documents/Updated%20critical%20concentration%20table_1st%20and%202nd%20line%20drugs.pdf Accessed 19 August 2015.
- 29. Jo KW, Lee SD, Kim WS, Kim DS, Shim TS. Treatment outcomes and moxifloxacin susceptibility in ofloxacin-resistant multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2014; 18:39–43. [DOI] [PubMed] [Google Scholar]
- 30. Falzon D, Gandhi N, Migliori GB et al. ; Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J 2013; 42:156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cegielski JP, Kurbatova E, van der Walt M et al. ; Global PETTS Investigators Multidrug-resistant tuberculosis treatment outcomes in relation to treatment and initial versus acquired second-line drug resistance. Clin Infect Dis 2016; 62:418–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim HR, Hwang SS, Kim HJ et al. . Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis 2007; 45:1290–5. [DOI] [PubMed] [Google Scholar]
- 33. Lin CH, Lin CJ, Kuo YW et al. . Tuberculosis mortality: patient characteristics and causes. BMC Infect Dis 2014; 14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhargava A, Chatterjee M, Jain Y et al. . Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLoS One 2013; 8:e77979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pednekar MS, Hakama M, Gupta PC. Tobacco use or body mass—do they predict tuberculosis mortality in Mumbai, India? Results from a population-based cohort study. PLoS One 2012; 7:e39443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kwon YS, Kim YH, Suh GY et al. . Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis 2008; 47:496–502. [DOI] [PubMed] [Google Scholar]
- 37. Tang S, Tan S, Yao L et al. . Risk factors for poor treatment outcomes in patients with MDR-TB and XDR-TB in China: retrospective multi-center investigation. PLoS One 2013; 8:e82943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gler MT, Guilatco R, Caoili JC, Ershova J, Cegielski P, Johnson JL. Weight gain and response to treatment for multidrug-resistant tuberculosis. Am J Trop Med Hyg 2013; 89:943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]