Summary
Increased ganciclovir exposure is a risk factor for ganciclovir-resistant cytomegalovirus (ganR-CMV) development, and ganR-CMV is associated with increased attributable morbidity and mortality. Our data also suggest that testing thresholds for prior drug exposure in current consensus guidelines may need to be revised.
Keywords: cytomegalovirus, solid organ transplant, ganciclovir resistance, risk factors, outcomes
Abstract
Background
Ganciclovir-resistant (ganR) cytomegalovirus (CMV) is an emerging and important problem in solid organ transplant (SOT) recipients. Only through direct comparison of ganR- and ganciclovir-sensitive (ganS) CMV infection can risk factors and outcomes attributable specifically to ganciclovir resistance appropriately be determined.
Methods
We performed a retrospective, case-control (1:3) study of SOT recipients with genotypically confirmed ganR-CMV (n = 37) and ganS-CMV infection (n = 109), matched by donor/recipient CMV serostatus, year and organ transplanted, and clinical manifestation. We used χ2 (categorical) and Mann-Whitney (continuous) tests to determine predisposing factors and morbidity attributable to resistance, and Kaplan-Meier plots to analyze survival differences.
Results
The rate of ganR-CMV was 1% (37/3467) overall and 4.1% (32/777) among CMV donor-positive, recipient-negative patients, and was stable over the study period. GanR-CMV was associated with increased prior exposure to ganciclovir (median, 153 vs 91 days, P < .001). Eighteen percent (3/17) of lung transplant recipients with ganR-CMV had received <6 weeks of prior ganciclovir (current guideline-recommended resistance testing threshold), and all non-lung recipients had received ≥90 days (median, 160 [range, 90–284 days]) prior to diagnosis of ganR-CMV. GanR-CMV was associated with higher mortality (11% vs 1%, P = .004), fewer days alive and nonhospitalized (73 vs 81, P = .039), and decreased renal function (42% vs 19%, P = .008) by 3 months after diagnosis.
Conclusions
GanR-CMV is associated with longer prior antiviral duration and higher attributable morbidity and mortality than ganS-CMV. Upcoming revised CMV guidelines should incorporate organ transplant–specific thresholds of prior drug exposure to guide rational ganR-CMV testing in SOT recipients. Improved strategies for prevention and treatment of ganR-CMV are warranted.
Ganciclovir-resistant (ganR) cytomegalovirus (CMV) infection in solid organ transplant (SOT) recipients has been increasingly reported, particularly with the use of more potent immunosuppression and increased durations of antiviral drug exposure. Prior studies have reported that the incidence of ganR-CMV is associated with type of organ transplanted, CMV serostatus of the recipient and the donor, lower doses or longer duration of ganciclovir prophylaxis, high CMV viral loads, and more intensive immunosuppression [1–8]. Additionally, ganciclovir resistance has been associated with longer hospitalization and increased mortality [5]. Important limitations of these studies have included small patient numbers, limited information on clinical outcomes, and cohort study designs that compared patients with ganR-CMV to all other patients (including those without CMV infection). With these cohort study designs, the risk factors and outcomes that have been reported to be associated with ganR-CMV could not be directly attributed to ganciclovir resistance. Thus, a study design that directly compares ganR-CMV and ganciclovir-sensitive (ganS) CMV is crucial to identify risk factors and outcomes that can be specifically attributed to ganciclovir resistance.
Only 4 small prior studies included direct comparisons of patients with ganR- to those with ganS-CMV. Young et al focused on abdominal organ recipients and the association of ganciclovir resistance with alemtuzumab use. The sample size was small (10 patients with genotypically confirmed ganR-CMV), and their findings were predominantly descriptive [9]. Timpone et al looked at ganR-CMV vs ganS-CMV in intestinal and multivisceral organ transplant recipients, but only had 4 genotypically confirmed ganR-CMV cases and did not assess ganciclovir duration as a potential risk factor for resistance [10]. Bhorade et al compared survival in 12 lung transplant recipients with genotypically confirmed ganR-CMV to patients with ganS-CMV and found increased mortality associated with ganR-CMV [3]. Kruger et al also examined risk factors of ganR-CMV in 18 lung transplant recipients and 18 controls (14 controls with CMV viremia); however, cases were not genotypically confirmed, and survival outcomes were compared between ganR-CMV and all other lung transplant recipients, not ganS-CMV controls, precluding the ability to assess attributable impact of resistance on mortality [8].
To address some of these important limitations of prior studies, we performed a large case-control study of genotypically confirmed ganR-CMV cases matched to ganS-CMV controls to examine risk factors and outcomes in SOT recipients that could be specifically attributable to ganciclovir resistance. A better understanding of risk factors and outcomes of ganR-CMV is an important first step in developing better preventive and therapeutic strategies. Additionally, better defining the incidence of these infections and outcomes is important for the rational design of future trials of preventive and treatment strategies for ganR-CMV.
METHODS
Cohorts
We retrospectively identified 37 adult patients transplanted between 1993–2010 at the University of Washington Medical Center who received a lung, heart, kidney, pancreas, or liver transplant and had genotypically confirmed ganR-CMV infection. Two independent data abstractors used standardized data collection forms to identify the cases, and discrepancies were resolved by primary review by an author (A. P. L.). We matched the ganR-CMV case patients approximately 1:3 to 109 adult transplant recipients who developed ganS-CMV infection during the same time period. Patients were identified through review of a prospectively maintained clinical database of SOT recipients with CMV infection/disease as previously described [11]. Patients were matched by CMV donor/recipient serostatus (CMV donor positive, recipient negative [D+R−]; donor negative, recipient negative [D−R−]; and recipient positive [R+]), type of organ transplanted, year of transplant ±3 years, and CMV disease type (refractory viremia, CMV syndrome, tissue invasive). CMV disease definitions were adapted from published guidelines [12, 13]: Patients with compatible symptoms and CMV demonstrated in biopsy specimens by either isolation of CMV by culture or histopathology immunohistochemistry were considered to have tissue-invasive disease. Patients with symptoms and CMV viremia who did not meet tissue-invasive criteria were considered to have CMV syndrome, and patients with CMV viremia and no symptoms were considered to have asymptomatic viremia. Patient demographics, transplant details, and clinical and laboratory information were collected from comprehensive electronic health records. This study was approved by the University of Washington Institutional Review Board. Some clinical details of some of the ganR-CMV cases in this article were included in previous studies from our institution [2, 4, 6, 14]; however, none of these studies systematically addressed the attributable risk factors for, or impact of, ganR-CMV using a case-control study design.
Immunosuppression, Rejection Therapy, and CMV Prophylaxis
CMV preventive strategies, immunosuppression regimens, and rejection treatment varied based on organ transplanted and time period, as described previously [11]. In brief, CMV prophylaxis generally included either oral ganciclovir (1 g 3 times daily) or valganciclovir (900 mg daily), both adjusted for renal function as per manufacturer recommendations. Duration of prophylaxis was generally 3 months for patients who were CMV R+ and 3–6 months for D+R− patients. D−R− recipients received acyclovir prophylaxis at a dose of 400 mg twice daily for at least 3 months posttransplant.
CMV Diagnostic Testing
CMV viremia was diagnosed by either detection of pp65 antigen (earlier time period of study) or quantitative polymerase chain reaction (PCR) in blood (more recent). Pp65 antigenemia was assessed via a commercially available kit, and PCR testing was done by a laboratory-developed real-time assay, as previously described [15].
Determination of CMV Resistance
Ganciclovir resistance testing was performed when clinically suspected, as previously described [4, 16]. In brief, indications for resistance testing included failure to achieve ≥1 log reduction in CMV viral load despite ≥2 weeks of appropriate ganciclovir or valganciclovir treatment, or failure to have a significant improvement in clinical symptoms despite 2 weeks of full-dose ganciclovir or valganciclovir therapy. CMV genotypic resistance testing was performed using well-validated assays, as described previously [17, 18], and interpretation of UL97 and UL54 mutations conferring ganciclovir resistance was done as previously published [19].
Statistical Analysis
We examined the association between different variables (eg, age, sex, duration of ganciclovir/valganciclovir exposure) and development of ganR-CMV infection. We also assessed the outcomes of ganR-CMV infection, compared with matched controls with ganS-CMV infection. The primary clinical outcome was mortality (3 and 12 months following CMV diagnosis), and secondary clinical endpoints included decrease in kidney function (defined as a ≥20% decrease in estimated glomerular filtration rate [eGFR] at 3 months after CMV diagnosis), number of days alive and not hospitalized in the 3 months following CMV diagnosis (“well days”), and acute allograft rejection in the first year following CMV. The χ2 and Fisher exact (categorical variables) and Mann-Whitney (continuous variables) tests were used to compare ganR and ganS groups. The Kaplan-Meier method was used to estimate survival, and the log-rank test was used to compare survival curves. P values <.05 were considered statistically significant. All analyses were performed using Stata software version 13.1 (StataCorp, College Station, Texas).
RESULTS
Study Population (Cases and Controls)
We identified 37 SOT recipients with genotypically confirmed ganR-CMV infection between 1993 and 2010, and 109 matched SOT recipients with ganS-CMV. Table 1 shows that the cases and controls were appropriately matched on the selected variables: CMV serostatus, organ transplanted, CMV manifestation, and year of transplantation. All ganR-CMV cases had 1 or more UL97 mutations and 2 cases had an additional UL54 mutation known to confer phenotypic resistance to ganciclovir (Table 2).
Table 1.
Details of the Matching of Cases and Controlsa
| Characteristic | GanR-CMV Cases (n = 37), No. (%) | GanS-CMV Controls (n = 109), No. (%) | P Value |
|---|---|---|---|
| CMV serostatus | |||
| D+/R− | 32 (86.5) | 97 (89.0) | .85 |
| D−/R− | 3 (8.1) | 6 (5.5) | |
| R+ | 2 (5.4) | 6 (5.5) | |
| Organ transplanted | |||
| Lung | 17 (45.6) | 52 (47.7) | .95 |
| Heart | 6 (16.2) | 20 (18.4) | |
| Kidney | 6 (16.2) | 19 (17.4) | |
| Pancreas | 6 (16.2) | 12 (11.0) | |
| Liver | 2 (5.4) | 6 (5.5) | |
| CMV manifestation | |||
| Refractory viremia | 7 (18.9) | 22 (20.2) | .99 |
| Syndrome | 11 (29.7) | 32 (29.4) | |
| Tissue-invasive | 19 (51.4) | 55 (50.5) | |
Abbreviations: CMV, cytomegalovirus; D, donor; ganR, ganciclovir resistant; ganS, ganciclovir sensitive; R, recipient.
aAll controls were also matched to cases by year of transplant ±3 years.
Table 2.
Specific UL97 and UL54 Mutations in Patients With Ganciclovir-Resistant Cytomegalovirus
| Mutation | No.a |
|---|---|
| UL97 mutations | |
| L595S | 11 |
| L595F | 2 |
| L595W | 1 |
| A594V | 8 |
| A594T | 1 |
| H520Q | 4 |
| M460V | 3 |
| M460I | 3 |
| C603W | 3 |
| DEL600-1 | 1 |
| DEL599-603 | 1 |
| UL54 mutations | |
| F412Cb | 1 |
| P522Sc | 1 |
aTotal is 40 as some patients had 2 mutations.
bPatient only had a UL54 mutation (no UL97).
cPatient also had a UL97 mutation (L595S).
The incidence of ganR-CMV in all SOT recipients (regardless of serostatus) was 1.0% overall (37 cases among 3647 SOT recipients during the study period) and 4.1% (32/777) in D+R− patients. Among the D+R− patients, the incidence of ganR-CMV in lung, heart, kidney, pancreas, and liver recipients was 11.9%, 5.8%, 2.4%, 7.8%, and 0.4%, respectively. There were no significant changes in the incidence of ganR-CMV infection over the study period: For the time periods 1993–1998, 1999–2004, and 2005–2010 the incidence was1.1%, 1.0%, and 1.0% when including all serostatuses, and 5.2%, 4.6%, and 3.3% in the D+R− subset, respectively.
Factors Associated With Development of GanR-CMV Infection
We examined several patient and transplant variables as potential risk factors for development of ganR-CMV vs ganS-CMV infection (Table 3). Among 22 of 37 (59%) of the cases for whom data was available, all developed ganR during their first viral episode. In all patients, we assessed cumulative receipt of any form of ganciclovir (oral ganciclovir, intravenous ganciclovir, and valganciclovir) prior to diagnosis and found that a longer total duration was significantly associated with development of ganR-CMV. The median exposure (range) in all ganR-CMV cases, in the subset of lung transplant only, and in non-lung transplant ganR-CMV cases is shown in (Table 4). Lung transplant recipients received significantly less ganciclovir prior to ganR-CMV diagnosis; additionally, 3 of 17 (17.6%) of lung transplant recipients developed ganR-CMV prior to 6 weeks (after 30, 35, and 40 days) of ganciclovir exposure (the minimum recommended duration of prior prophylactic drug exposure to warrant testing for ganR in current CMV international guidelines) [16]. In contrast, none of the non-lung transplant recipients developed ganR-CMV prior to 6 weeks of ganciclovir/valganciclovir (earliest onset was after 90 days of drug exposure, followed by 124 days). The mean and median peak CMV viral loads were also significantly higher in cases than controls (Table 3).
Table 3.
Factors Associated With Development of Ganciclovir-Resistant Cytomegalovirus
| Characteristic | Cases (n = 37) | Controls (n = 109) | P Value |
|---|---|---|---|
| Median age at CMV diagnosis (IQR) | 57.7 (47.3–62.4) | 53.2 (38.1–62.8) | .23 |
| Male sex | 28 (75.7) | 63 (57.8) | .052 |
| Race, white | 35 (94.6) | 102 (93.6) | .82 |
| Induction immunosuppressiona | |||
| Yes | 31 (86.1) | 81 (86.2) | .99 |
| No | 5 (13.9) | 13 (13.8) | |
| Induction immunosuppression type | |||
| Antilymphocyte antibody | 17 (54.8) | 38 (46.9) | .45 |
| IL-2 receptor antagonist | 14 (45.2) | 43 (53.1) | |
| Median days to CMV diagnosis posttransplant (IQR) | 196 (147–300) | 143 (112–230) | .059 |
| Median ganciclovir exposure prior to CMV diagnosis, d (IQR)b | 153 (121–208) | 91 (41–108) | <.001 |
| Mean peak viral load (SD), IU/mLc | 266393 (768202) | 56560 (130818) | .037 |
| Median peak viral load (IQR), IU/mLc | 61250 (30000–142500) | 8125 (1913–37500) | <.001 |
| Rejection within 3 months prior to CMV diagnosis | 8 (21.6) | 26 (23.9) | .78 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CMV, cytomegalovirus; D, donor; IL-2, interleukin 2; IQR, interquartile range; R, recipient; SD, standard deviation.
aSixteen patients with unknown induction status.
bExposure of ganciclovir (oral or intravenous) and/or valganciclovir prior to diagnosis of either ganciclovir-sensitive or ganciclovir-resistant CMV as applicable.
cPrior to ganciclovir resistance diagnosis in cases; information on this was found in 22 of 37 cases and 65 of 109 controls.
Table 4.
Days of Ganciclovir/Valganciclovir Received Prior to Development of Ganciclovir-Resistant Cytomegalovirus in Patients by Type of Organ Transplanted
| Organ Transplanted | Days of Ganciclovir/Valganciclovir Received, Median (Range)a | P Value |
|---|---|---|
| All organs (n = 37) | 153 (30–284) | |
| Lung (n = 17) | 121 (30–269) | .02 |
| Non-lung (n = 20) | 160 (90–284) |
aFull range (not interquartile) used to show the earliest resistance development.
Treatment of GanR-CMV Infection
Treatment information was available for 35 of 37 (95%) ganR-CMV patients, and 24 (69%) received foscarnet. Other treatments included reduction in immunosuppression (as feasible), conversion to mammalian target of rapamycin (mTOR) inhibitor–based immunosuppression, use of CMV hyperimmune globulin, and/or higher than standard-dose intravenous ganciclovir.
Outcomes of GanR-CMV Infection
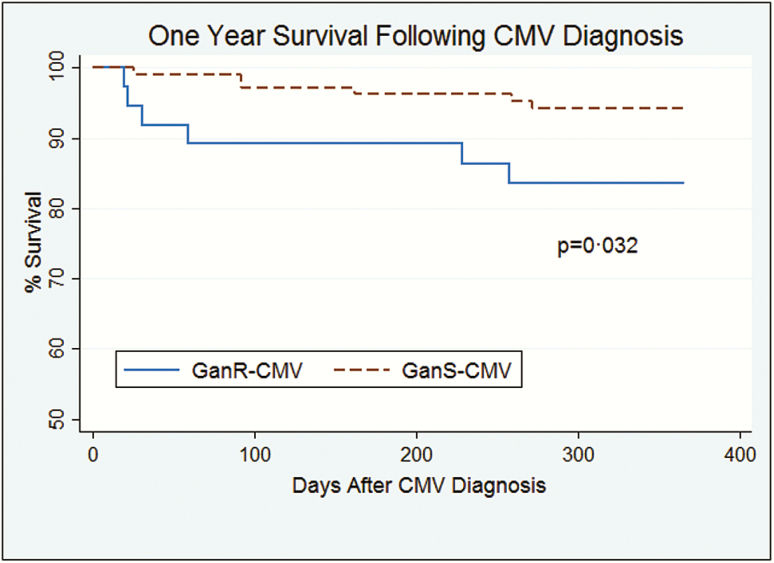
We compared virologic parameters (time to virus clearance), morbidity (number of days well and free from hospitalization, renal function, rejection), and mortality outcomes between those with ganR-CMV vs ganS-CMV. Table 5 shows these outcomes, and Figure 1 shows the Kaplan-Meier survival curves for mortality at 1 year. In cases, time to virus clearance refers to the ganR-CMV episode, and in controls it refers to the first CMV episode. At the end of last follow-up (median, 48 [interquartile range {IQR}, 23–78] months for cases and 41 [IQR, 22–76] months for controls, P = .83), 14 (37.8%) patients with ganR-CMV and 26 (23.9%) patients with ganS-CMV had died. The majority of deaths in the ganR-CMV group (67%) occurred within 3 months, as compared to 11% in the ganS-CMV group, demonstrating that the greatest risk of death was proximate to the diagnosis of ganR-CMV, compatible with attributable mortality. Patients with ganR-CMV had worse outcomes in all criteria examined except acute allograft rejection in the first year after diagnosis. While acute allograft rejection in the first year was not significantly increased in the entire cohort of SOT recipients with ganR-CMV, an increased rate of acute allograft rejection was observed in the subset of kidney recipients with ganR-CMV (Table 5). We further analyzed the association between eGFR decrease at 3 months and ganR-CMV, and found that significantly more patients with ganR-CMV had a ≥20% decrease of eGFR (Table 5). This eGFR decrement was specifically associated with receipt of foscarnet: 54.2% of ganR-CMV patients who received foscarnet had a ≥20% decrease in eGFR at 3 months compared with 19.4% in controls (P < .001), whereas ganR-CMV patients who did not receive foscarnet had similar rates of eGFR reduction to ganS-CMV patients (20.0% vs 19.4%, P = .97).
Table 5.
Comparison of Outcomes in Patients With Ganciclovir-Resistant Versus Ganciclovir-Sensitive Cytomegalovirus
| Outcome | Cases (n = 37) | Controls (n = 109) | P Value |
|---|---|---|---|
| Morbidity measures | |||
| Days to clearance of viremia, median (IQR) | 113 (50–394) | 53 (32–149) | .006 |
| ≥20% decrease in eGFR by 3 mo after CMV diagnosis | 15 (41.7) | 21 (19.4) | .008 |
| Well daysa in the 3 mo after CMV diagnosis, mean (SE) | 72.7 (4.8) | 81.0 (1.7) | .039 |
| Rejection within 1 y following CMV diagnosis | |||
| All organs | 15 (40.5) | 38 (34.9) | .54 |
| Kidney | 4 (66.7) | 2 (10.5) | .005 |
| Mortality | |||
| 3 mo | 4 (10.8) | 1 (0.92) | .004b |
| 12 mo | 6 (16.2) | 6 (5.5) | .032 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate; IQR, interquartile range; SE, standard error.
aAlive and nonhospitalized.
bFisher exact test.
Figure 1.
Kaplan-Meier curve of survival following cytomegalovirus (CMV) diagnosis in transplant recipients with ganciclovir-resistant (GanR) CMV and ganciclovir-sensitive (GanS) CMV.
We separately examined the subset of lung transplant recipients, who accounted for the largest organ transplant subgroup with ganR-CMV. Both the risk factors and outcomes were generally qualitatively similar to those of the combined group of SOT recipients (Supplementary Tables 1 and 2), but differences between some of the associated factors and outcomes between the ganR- and ganS-CMV groups no longer reached statistical significance because of smaller numbers.
DISCUSSION
By utilizing a case-control study design, we determined the risk factors and outcomes attributable to genotypically confirmed ganciclovir resistance in a large cohort of SOT recipients. We demonstrated that development of ganciclovir resistance was significantly associated with receipt of longer prior duration of ganciclovir. Furthermore, we demonstrated that ganciclovir resistance is associated with significantly increased attributable morbidity (decreased days free from hospitalization, higher rate of renal dysfunction, and, in kidney transplant recipients, increased rejection) and mortality.
We examined several potential contributors to ganR-CMV development. CMV D+R− serostatus is a well-established risk factor for ganR-CMV development [2–5, 7], and is reflected in the high proportion of D+R− patients in the ganR-CMV case cohort, and the disproportionately high rate among D+R− SOT recipients in general. The rationale for matching cases and controls for this variable was that D+R− status is already well established as a risk factor, and matching for this variable would allow identification of other potentially modifiable factors that might predispose to ganciclovir resistance. We found that a longer duration of all forms of ganciclovir exposure was a significant risk factor for ganR-CMV, confirming and extending results found in previous smaller studies [1–6, 8]. If development of ganR-CMV is a direct effect of a longer duration of ganciclovir, effective CMV prevention strategies that minimize drug exposure would be expected to lead to lower resistance rates, at least among high-risk D+R− patients. Alternatively, the need for a longer duration to control CMV may be a surrogate for inadequate CMV-specific immunity or other factors, and these should be specifically assessed in future studies. We found that an important proportion (3/17 [18%]) of lung transplant recipients developed resistance after <6 weeks of prior drug exposure. However, this finding was based on small numbers. If confirmed in other studies, this would provide important information about minimum prior drug exposure associated with subsequent resistance development and has 2 important implications. First, current CMV consensus guidelines recommending resistance testing after a minimum of 6 weeks of drug exposure may miss a significant proportion of ganR-CMV in lung transplant patients. And second, future guidelines should consider inclusion of organ transplant type-specific recommendations of minimal prior drug exposure (ie, lung vs non-lung transplant recipients) that should lead to resistance testing in patients who fail to have a clinical and/or virologic response to appropriately dosed ganciclovir therapy.
Previous studies have reported an association of more potent immunosuppression [4, 5, 7] or specific immunosuppressive drugs, such as anti-thymocyte globulin (ATG) or daclizumab [3, 8], with ganR-CMV. We did not find an association in this study. One possible explanation is the high rate of induction therapy in this cohort, thereby limiting our ability to assess this as a risk factor. Alternatively, while more potent immunosuppression or induction immunosuppression might be risk factors for CMV infection/disease in general, they might not necessarily increase the risk for ganR-CMV above and beyond the risk for CMV in general.
An important unresolved issue has been whether ganciclovir resistance is truly associated with attributable morbidity or mortality above and beyond ganciclovir-susceptible CMV. Prior studies that used non–case-control study designs or small case numbers were limited in their ability to determine the attributable impact of ganciclovir resistance on morbidity or mortality. In the present study, through the use of a robust case-control study design with adequate numbers of patients, we determined that ganciclovir resistance was associated with significantly increased attributable morbidity and mortality beyond that of ganS-CMV. GanR-CMV patients had increased mortality at both 3 months (10.8% vs 0.92% in matched ganS-CMV controls) and 12 months (16.2% vs 5.5%) after diagnosis. Increased mortality among cases was most evident in the first 3 months, consistent with a direct attributable effect of ganciclovir resistance on mortality. These data suggest that future studies of new treatments for ganR-CMV incorporate this important clinical endpoint into study designs.
GanR-CMV was also associated with increased morbidity measures, including longer duration of hospitalization and decreased renal function in the 3 months following ganR- or ganS-CMV diagnosis. A recent study by Avery et al examined the outcomes associated with receipt of foscarnet specifically, reporting a high (>50%) rate of renal toxicity associated with this treatment [20], although this included both hematopoietic cell transplant and SOT recipients. Similarly, in our study we found that 54% of ganR-CMV patients who received foscarnet had worsened renal function at 3 months. GanR-CMV has also been associated with graft dysfunction/rejection: Kruger et al described an increase incidence of bronchiolitis obliterans syndrome in lung transplant recipients with ganR-CMV, although the comparison group was all lung transplant recipients [8]. In our study, rejection in the first year following ganR-CMV diagnosis was not statistically significantly higher than ganS-CMV when analyzing all organ recipients, but was significantly higher in the subset of kidney transplant recipients.
These data on attributable morbidity and mortality of ganciclovir resistance from our study provide important background information and endpoints for the design of future studies of novel treatments for ganR-CMV.
Our study has several strengths. It is the largest to date to our knowledge, and the matched case-control study design allowed us to analyze the risk factors and outcomes directly attributable to ganciclovir resistance. Only patients with genotypically confirmed ganR-CMV were included. While other studies have reported poor outcomes in ganR-CMV patients, these studies have had small numbers of patients and, with the exception of Bhorade et al, have mainly used descriptive statistics rather than formal statistical comparisons of ganR- to ganS-CMV–infected patients [3]. Our study also has potential weaknesses. Data on indication for ganciclovir use (prophylaxis vs treatment) were not collected, and so we were unable to directly examine this. Additionally, as commercial assays for blood ganciclovir levels were not widely available and were not performed during clinical care for our patients, we were unable to collect details on therapeutic vs subtherapeutic ganciclovir levels. Both of these issues should be addressed in future studies. Due to the relatively uncommon occurrence of ganR-CMV infection, our study was retrospective to maximize case numbers. Cases therefore spanned a long time period during which transplant practices have changed; however, this issue was addressed through matching by year of transplantation, and we found that the rates of ganR CMV did not change significantly over the study period. We also did not include any intestine/multivisceral transplant recipients, but this represents a very small number of transplants worldwide. As a single-center study, caution should be used when generalizing these results to other transplant centers with differing immunosuppression or other transplant practices.
In summary, using a case-control design, we identified longer duration of antiviral use as a significant predisposing factor for development of ganR-CMV in SOT recipients. Furthermore, we demonstrated that ganR-CMV has a significant attributable negative clinical impact beyond ganciclovir-susceptible CMV alone, and therefore merits improved prevention and treatment strategies. These results identify patients at greatest risk for developing ganR-CMV, provide important background data for the rational design of preventive approaches and interventional trials of novel agents for treatment of ganR-CMV, and impact future CMV consensus guidelines. If other studies of ganR-resistant CMV in lung vs non-lung transplant recipients confirm our findings, then current CMV guidelines for ganciclovir resistance testing should be revised into organ-specific recommendations (ie, lung vs non-lung transplant recipients) and the duration of prior drug exposure that should trigger resistance testing revised accordingly. Better strategies to prevent and treat ganR-CMV have the potential to improve clinically meaningful outcomes in SOT recipients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. C. E. F.: literature search, study design, data collection, statistical/data analysis and interpretation, writing. A. P. L.: literature search, study design, data collection, data analysis and interpretation, writing. J. K.: literature search, study design, data collection, writing. M. B.: study design, data interpretation and analysis, writing. E. D. L., K. J., R. R.: literature search, data interpretation, writing.
Acknowledgments. We are indebted to Michaela Kusumi, Fatima Ali, and Yodit Tekle for expert assistance with data abstraction and regulatory compliance.
Financial support. This work was supported by the National Institutes of Health (grant number K24HL093294 to M. B.) and the Joel Meyers Endowment Scholarship (to C. E. F.).
Potential conflicts of interest. M. B. consults and receives research support from Merck, Chimerix Inc, Astellas, Shire, and Gilead Sciences, and also consults for Microbiotix and Helocyte. A. P. L. consults and receives research support from Merck, Astellas, and Helocyte; has served as site investigator for clinical trials sponsored by Astellas, Shire, and Gilead; and consults for Helocyte and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin Transplant 2008; 22:162–70. [DOI] [PubMed] [Google Scholar]
- 2. Li F, Kenyon KW, Kirby KA, Fishbein DP, Boeckh M, Limaye AP. Incidence and clinical features of ganciclovir-resistant cytomegalovirus disease in heart transplant recipients. Clin Infect Dis 2007; 45:439–47. [DOI] [PubMed] [Google Scholar]
- 3. Bhorade SM, Lurain NS, Jordan A, et al. Emergence of ganciclovir-resistant cytomegalovirus in lung transplant recipients. J Heart Lung Transplant 2002; 21:1274–82. [DOI] [PubMed] [Google Scholar]
- 4. Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 2000; 356:645–9. [DOI] [PubMed] [Google Scholar]
- 5. Limaye AP. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin Infect Dis 2002; 35:866–72. [DOI] [PubMed] [Google Scholar]
- 6. Limaye AP, Raghu G, Koelle DM, Ferrenberg J, Huang ML, Boeckh M. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J Infect Dis 2002; 185:20–7. [DOI] [PubMed] [Google Scholar]
- 7. Minces LR, Nguyen MH, Mitsani D, et al. Ganciclovir-resistant cytomegalovirus infections among lung transplant recipients are associated with poor outcomes despite treatment with foscarnet-containing regimens. Antimicrob Agents Chemother 2014; 58:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kruger RM, Shannon WD, Arens MQ, Lynch JP, Storch GA, Trulock EP. The impact of ganciclovir-resistant cytomegalovirus infection after lung transplantation. Transplantation 1999; 68:1272–9. [DOI] [PubMed] [Google Scholar]
- 9. Young PG, Rubin J, Angarone M, et al. Ganciclovir-resistant cytomegalovirus infection in solid organ transplant recipients: a single-center retrospective cohort study. Transpl Infect Dis 2016; 18:390–5. [DOI] [PubMed] [Google Scholar]
- 10. Timpone JG, Yimen M, Cox S, et al. Resistant cytomegalovirus in intestinal and multivisceral transplant recipients. Transpl Infect Dis 2016; 18:202–9. [DOI] [PubMed] [Google Scholar]
- 11. Fisher CE, Alexander J, Bhattacharya R, et al. Sensitivity of blood and tissue diagnostics for gastrointestinal cytomegalovirus disease in solid organ transplant recipients. Transpl Infect Dis 2016; 18:372–80. [DOI] [PubMed] [Google Scholar]
- 12. Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34:1094–7. [DOI] [PubMed] [Google Scholar]
- 13. Humar A, Michaels M; AST ID Working Group on Infectious Disease Monitoring American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant 2006; 6:262–74. [DOI] [PubMed] [Google Scholar]
- 14. Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation 2006; 81:1645–52. [DOI] [PubMed] [Google Scholar]
- 15. Boeckh M, Huang M, Ferrenberg J, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol 2004; 42:1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kotton CN, Kumar D, Caliendo AM, et al. ; Transplantation Society International CMV Consensus Group Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013; 96:333–60. [DOI] [PubMed] [Google Scholar]
- 17. Castor J, Cook L, Corey L, Jerome KR. Rapid detection directly from patient serum samples of human cytomegalovirus UL97 mutations conferring ganciclovir resistance. J Clin Microbiol 2007; 45:2681–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hall Sedlak R, Castor J, Butler-Wu SM, et al. Rapid detection of human cytomegalovirus UL97 and UL54 mutations directly from patient samples. J Clin Microbiol 2013; 51:2354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chou S. Approach to drug-resistant cytomegalovirus in transplant recipients. Curr Opin Infect Dis 2015; 28:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Avery RK, Arav-Boger R, Marr KA, et al. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection. Transplantation 2016; 100:e74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.