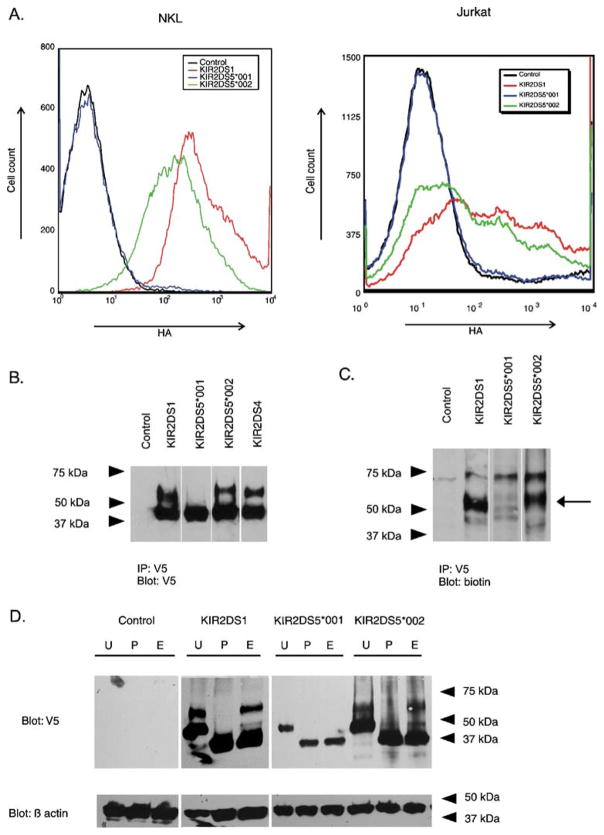
Fig. 3.
Flow cytometry and gel electrophoresis of stimulatory KIR show differences in cell surface expression and in the presence of receptor isoforms. a Representative flow cytometric analysis of transient transfectants expressing N-terminally HA-tagged, C-terminally V5-tagged KIR constructs. The tags facilitated the measurement of surface expression (HA) and total KIR protein (V5). The histogram shows the fluorescence intensity (HA) for receptors encoded by KIR2DS5*001 and KIR2DS5*002 in NKL (left) and in Jurkat (right) compared to a similarly tagged KIR2DS1 positive control after gating on the V5-positive populations. The negative control expressed a KIR2DS molecule without an HA tag. b Isolation of C-terminally V5-tagged KIR by immunoprecipitation from transfected NKL cells was followed by gel electrophoresis and Western blotting with a V5-specific Ab. Cells transfected with empty vector served as a negative control. Two isoforms were detected for KIR2DS1, KIR2DS4, and KIR2DS5 (*002). A single isoform with mobility similar to the lower molecular weight band exhibited by the other transfectants was detected for cells transfected with KIR2DS5*001. c Transfected cells were lysed after surface biotinylation. Proteins were isolated by immunoprecipitation and characterized by gel electrophoresis and Western blotting. A probe specific for biotinylated protein detected the higher molecular weight isoforms of KIR2DS1 and KIR2DS5 (*002) as indicated by the arrow. d NKL cells were transfected with KIR constructs carrying a V5-tag and the lysates were mock digested (U) or digested with PNGase F (P) or endoglycosidase H (E). Lysates were analyzed by Western blotting using the V5-specific Ab. Control indicates NKL cells transfected with an empty vector. Beta actin served as a loading control. PNGase F digested both isoforms of KIR2DS5 (*002) and KIR2DS1 to a core protein of one molecular mass. The lower molecular mass forms, but not the higher molecular mass forms, of KIR2DS5 (*002) and KIR2DS1 were susceptible to endoglycosidase H digestion. In contrast, the single isoform observed for KIR2DS5 (*001) was susceptible to both treatments