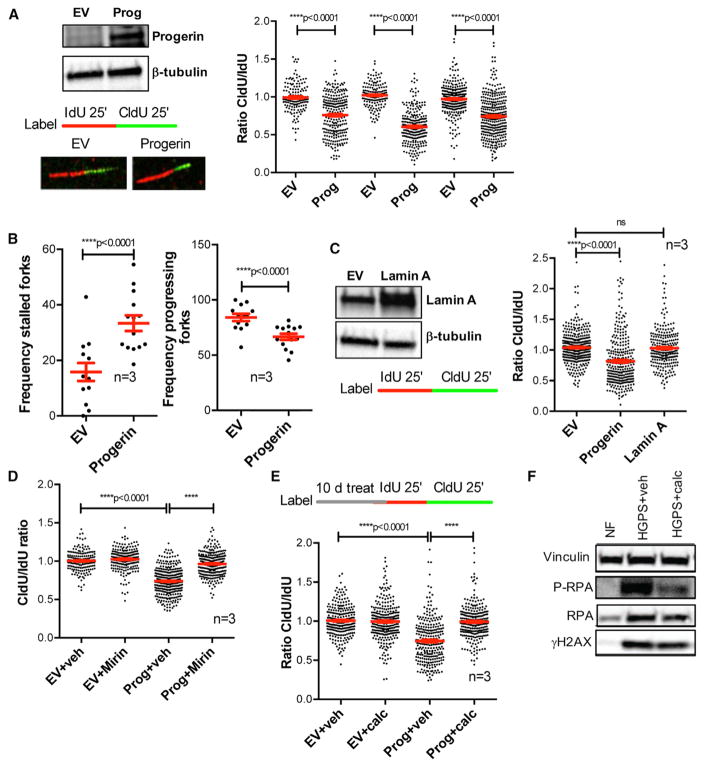
Figure 1. Replication Stress (RS) in Progerin-Expressing Cells.
(A) RPE cells were lentivirally transduced with progerin or empty vector (EV), and single-molecule replication analysis was performed. Immunoblots show the expression levels of progerin. Images show the incorporation of thymidine analogs in cells labeled with IdU for 25 min + CldU for 25 min, as detected by fluorescence confocal microscopy. Tract lengths are measured using ImageJ. Graph shows the tract length ratio CldU/IdU, which in control cells is 1 and in progerin-expressing cells is lower, indicating RF progression defects. Each experiment represents a biological repeat, with independent progerin expression. In each experiment, 200–250 forks were measured.
(B) The frequencies of stalled forks (only IdU signal) and progressing forks (both IdU and CldU signals) were counted in the three independent experiments in (A).
(C) DNA fibers were performed in RPE cells transduced with lamin A, progerin, or EV constructs. Graph shows the average ratio CldU/IdU in three biological repeats.
(D) DNA fiber assays performed with the same labeling scheme as in (A) in progerin-expressing and control RPE cells and treated with vehicle or mirin (5 μM) for 3 hr to inhibit Mre11 nuclease.
(E) Progerin-expressing and control RPE cells were treated with calcitriol (100 nM) for 10 days, and DNA fiber assays were performed as in (A).
(F) Untreated NFs and HGPS fibroblasts treated with vehicle or calcitriol for 4 days were processed for immunoblotting to monitor the levels of phosphorylated RPA (P-RPA) and H2AX (γH2AX). Vinculin was used as loading control.
* denotes p value of statistical significance (*p < 0.05, **p < 0.01, ***p < 0.005, and ****p < 0.001). Error bars represent SEM.