Figure 1.
Salicylate Inhibits EP300 Acetyltransferase by Competing with AcCoA
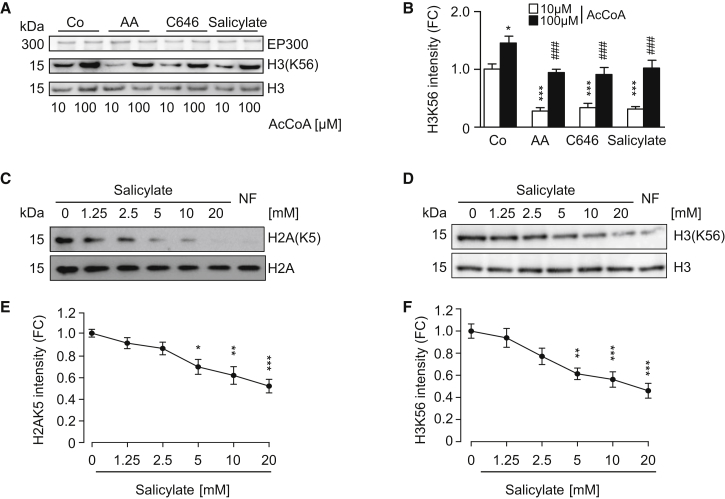
(A and B) Direct inhibition of EP300 acetyltransferase activity by salicylate. Recombinant EP300 protein was incubated with its substrate histone H3 in the presence of AcCoA, salicylate (5 mM), anacardic acid (AA, 50 μM), or C646 (10 μM), followed by immunoblotting to detect H3 acetylation on lysine 56 (A) and quantification (B) of 4 independent experiments (means ± SEM; ∗p < 0.05 and ∗∗∗p < 0.001, one-way ANOVA compared to 10 μM AcCoA control group; ###p < 0.001, one-way ANOVA compared to 100 μM AcCoA; FC, fold change).
(C and D) Salicylate inhibits EP300 activity toward its natural substrates. Human colorectal cancer HCT116 (C) and human osteosarcoma U2OS cells (D) were incubated for 16 hr with the indicated concentration of sodium salicylate and subjected to immunoblotting to evaluate H2A acetylation on lysine 5 (C) and H3 acetylation on lysine 56 (D) (quantified in E and F). Nutrient-free (NF) medium was used as a negative control of acetylation. Representative images of one experiment are shown.
(E and F) Quantification of data depicted in (C) and (D) (means ± SEM; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001, one-way ANOVA compared to control group).