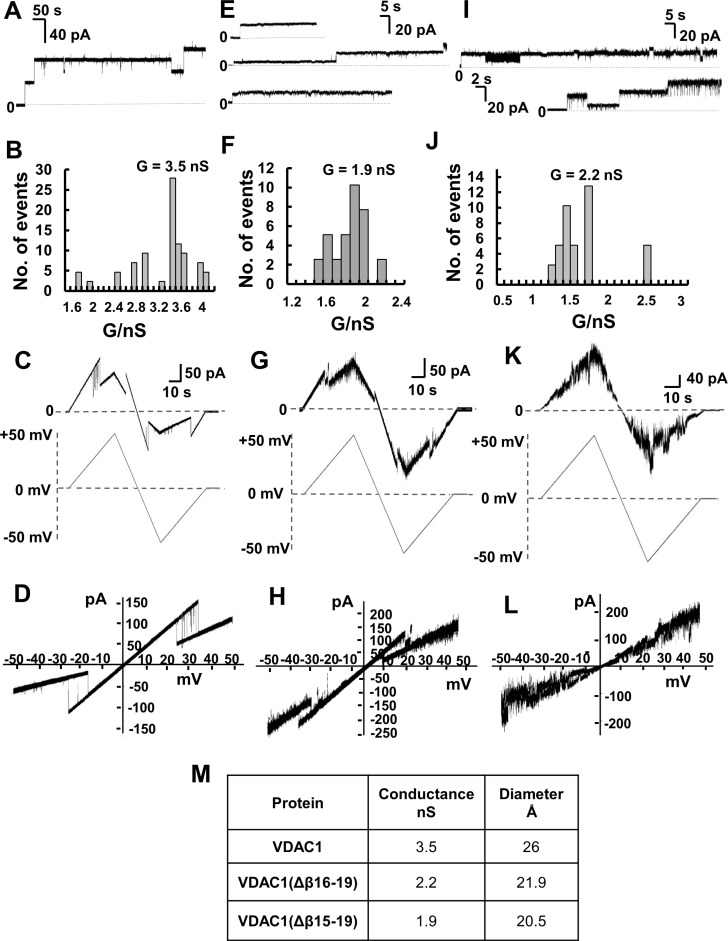
Figure 6. Channel properties of recombinant full-length and C-terminally truncated VDAC1 proteins.
Channel properties of recombinant and refolded full-length VDAC1, VDAC1(Δβ15-19) and VDAC1(Δβ16-19) reconstituted into a PLB. (A) Representative current traces of VDAC1 reconstituted into a DiphPC/n-decane phospholipid membrane recorded at 10 mV in 1 M KCl, 10 mM HEPES, pH 7.0, at 25° C. (B) Histogram of VDAC1 current amplitudes at 10 mV. The mean single channel conductance value was 3.5 nS for 40 insertion events. (C) Current amplitude changes across a planar bilayer containing a single VDAC1 channel in response to a linear voltage ramping between +50 mV and –50 mV. The buffer in the baths on either side of the bilayer was 1 M KCl, 10 mM HEPES, pH 7.0. (D) Current–voltage relationship (I–V curve) for VDAC1. (E) Representative current traces of VDAC1(Δβ15-19) in a PLB. Conditions as in (A). (F) Histogram of current amplitudes at 10 mV of VDAC1(Δβ15-19). The mean single channel conductance value was 1.9 nS for 22 insertion events. (G) Current amplitude changes across a planar bilayer containing a single VDAC1(Δβ15-19) channel in response to a linear voltage ramping between +50 mV and –50 mV. Conditions as in (C). (H) Current–voltage relationship (I–V curve) for VDAC1(Δβ15-19). (β15-19). (I) Representative current traces of VDAC1(Δβ16-19) in PLB. Conditions as in (A). (J) Histogram of VDAC1(Δβ16-19) current amplitudes at 10 mV. The mean single channel conductance value was 2.2 nS for 19 insertion events. (K) Current amplitude changes across a planar bilayer containing a single VDAC1(Δβ16-19) channel in response to a linear voltage ramping between +50 mV and –50 mV. Conditions as in (C). (L) Current–voltage relationship (I–V curve) for VDAC1(Δβ16-19). (M) Summary of the conductance and protein diameters of the three VDAC1 versions studied in this work. The diameters of the truncated VDAC1 versions were calculated assuming that each of them presents the same pore conformation and that each strand contributes 4.3 Å to the circumference. This figure was calculated from available VDAC1 structures, which comprise 19 β-strands, resulting in a pore circumference of 81.7 Å and a diameter of 26.0 Å (measured between Ser46 and Asn185) [63–65]. Accordingly, the calculated diameter of VDAC1(Δβ16-19] is 21.9 Å and 20.5 Å for VDAC1(Δβ15-19).