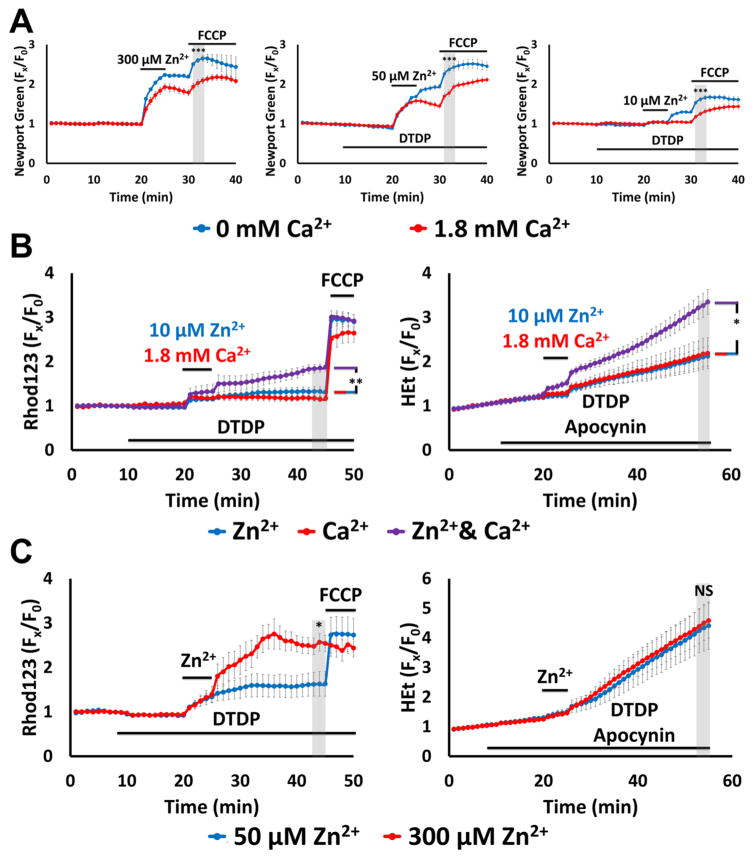
Figure 5. Ca2+ attenuates mitochondrial Zn2+ accumulation despite exacerbating the consequent dysfunction.
Cultures were loaded with Newport Green, Rhod123 or HEt in 0 or 1.8 Ca2+ HSS, and exposed to high K+ with 300, 50,10 or 0 μM Zn2+ (as indicated, along with 10 μM MK-801, to inhibit Ca2+-entry via NMDA receptor activation); DTDP (60 μM), FCCP (1 μM) and/or apocynin (500 μM) were added as indicated. Traces represent mean ± SEM Fx/F0 values for each dye and represents ≥ 5 experiments consisting of ≥ 120 neurons. Grey bars indicate time points of comparison (NS indicates No Significance, * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, by two-tailed t-test [A, C] or by one-way ANOVA with Tukey post hoc [B]).
A). Presence of Ca2+ decreases neuronal and mitochondrial Zn2+ uptake: Note that presence of Ca2+ attenuated both cytosolic Zn2+ rise during the exposure and FCCP-induced mitochondrial Zn2+ release.
B). Ca2+ and Zn2+ synergistically induce mitochondrial dysfunction: Neurons loaded with Rhod123 (left) or HEt (right) were exposed to high K+/DTDP/MK-801 with 10 μM Zn2+ (blue), 1.8 mM Ca2+ (red) or with both Zn2+ and Ca2+ (purple) for 5 min, then washed as indicated. Apocynin was added to HEt-loaded neurons (right) to inhibit contributions from Ca2+-dependent NOX activation. Note that despite relatively little effects from Ca2+ and Zn2+ individually, together they induced significant mitochondrial dysfunction.
C). Overwhelming mitochondrial Zn2+ loading induces rapid mitochondrial depolarization: Neurons loaded with Rhod123 (left) or HEt (right) in 1.8 Ca2+ HSS were exposed to high K+/DTDP/MK-801/Ca2+, with 50 (blue) or 300 μM (red) Zn2+ for 5 min, followed by wash as indicated. Note that 300 μM Zn2+ induced greater loss of ΔΨm than 50 μM Zn2+, despite both inducing similar levels of ROS generation.