Abstract
People with schizophrenia-spectrum disorders (SSD) often experience impairments in non-social motivation. In this study, we extended this line of investigation by examining specific components of social motivation and the extent to which these components work together in people with and without a recent-onset SSD. Sixty-four people with a recent-onset SSD and 26 controls completed a task that allowed us to examine changes in anticipated pleasure, decisions to trust, and effort expenditure over the course of repeated interactions with positive or negative outcomes. Compared to controls, we found that people in the SSD group placed less trust, tended to anticipate less pleasure, and expended less effort to increase the likelihood of future interactions with positive outcomes. Further, in the SSD group, effort expenditure was not associated with either anticipated pleasure or decisions to trust. While there were no group differences in anticipated pleasure or trust placed during interactions with negative outcomes, people in the SSD group expended less effort to decrease to the likelihood of future interactions. Taken together, our findings suggest that people with a recent-onset SSD may experience both impairment and disconnection between various components of social motivation for interactions with positive outcomes. Implications for interventions for social engagement in people with SSD are discussed.
Keywords: schizophrenia, motivation, social interaction, anticipated pleasure, reward learning, effort expenditure
Introduction
People across the schizophrenia spectrum often experience greater levels of social isolation and difficulties forming social relationships (Fulford et al., 2013; Schlosser et al., 2014). While recent models (Barch and Dowd, 2010; Kring and Barch, 2014) have identified specific components of non-social motivation that are often impaired in people with schizophrenia, it is unclear whether similar impairments in components of social motivation may contribute to decreased social engagement. For instance, people with schizophrenia may have a positive social interaction, but fail to use this positive outcome to generate the drive for future social engagement, thus contributing to poorer social functioning that is common in the illness (Robertson et al., 2014). In this study, we examining three distinct, but related components of motivation for social interactions in people with and without a recent-onset SSD: anticipated pleasure, reward learning, and effort expenditure. By focusing early in the course of illness, we sought to better understand how components of social motivation work together, or breakdown during a period that is critical for social development (Crone & Dahl, 2012).
Components of Non-Social Motivation
To better understand the nature of motivational impairment in schizophrenia, researchers have begun to unpack deficits in motivation into distinct, but related components. One such component is the experience of pleasure, which can be parsed into consummatory (in the moment) and anticipatory components (Kring and Elis 2013). Compared to controls, people with schizophrenia often anticipate less pleasure from physical, non-social positively valenced stimuli (Kring and Elis, 2013; but see Frost and Strauss, 2016). Another distinct, but related component is reward learning, or the ability to use positive and negative outcomes to inform subsequent decision-making. Compared to controls, people across the schizophrenia spectrum have been shown to have difficulties learning from positive, but not negative non-social outcomes (e.g. monetary gains or losses; Strauss et al., 2011; Gold et al., 2012; Chang et al., 2016). More recently, studies have shown that people with chronic schizophrenia have impairments in effort-based decision-making compared to controls (Green et al., 2015), inefficiently allocating effort expenditure as evidenced by difficulty using information about reward magnitude and the probability or reward receipt to update effort-based decision-making (Gold et al., 2013; Barch et al., 2014; Treadway et al., 2015; McCarthy et al., 2016).
Components of Social Motivation
To date, research into the various components of social motivation in SSD has been fairly limited. Only two studies have investigated anticipated pleasure from social outcomes finding that compared to controls, people with SSD reported less anticipated pleasure from being included in a social interaction (Engel et al., 2016) and during interactions with smiling social partners and positive outcomes (Campellone and Kring, 2017). In both of these studies, there were no group differences in anticipated pleasure for social interactions with negative outcomes. For reward learning, a few recent studies have investigated how people with and without schizophrenia use positive and negative social interaction outcomes to inform decisions to trust during subsequent interactions with social partners (see Fett et al., 2015). These studies have shown that people with SSD have comparative difficulties in learning from rewarding interactions, as evidenced by difficulties using positive social interaction outcomes (i.e., reciprocation of trust by a social partner) to inform subsequent decisions to trust (Fett et al., 2016; Campellone et al., 2016). Learning from negative social interaction outcomes, however, appears to be intact (Fett et al., 2012; Campellone et al., 2016, but see Fett et al., 2016). Finally, for effort expenditure, a recent study (de la Asuncion et al., 2015) found no group differences in reaction time for approaching and avoiding (single joystick movement) emotional faces. Overall, these studies suggest that impairments in discrete components of social motivation in SSD may be specific to positive social outcomes. Furthermore, these studies have shown that impairments in components of social motivation are associated with poorer social functioning (Campellone et al., 2016; Campellone and Kring, 2017). In this study, we build on these findings by simultaneously investigating each of these components as well as examining the inter-relationships between components.
The Role of Emotion Displays
Social interactions also contain other sources of information that can be used to inform social motivation, such as a social partner’s emotional display. In research with healthy people, smiles have been shown to promote trust (Scharlemann et al., 2001), activate brain regions associated with anticipated pleasure (Rademacher et al., 2010) and facilitate learning trustworthy behavior (Heerey, 2014). Scowls, on the other hand, signal rejection (Heerdink et al., 2015) and for others to keep their distance (Marsh et al., 2005). Furthermore, there is a growing body of evidence that emotional displays can influence components of social motivation in people with schizophrenia, such as decisions to trust (Campellone, Fisher, and Kring, 2016), anticipated pleasure (Campellone and Kring, 2017), and effort expenditure (de la Asuncion, 2015). In this study, we sought to add to this growing body of evidence by investigating potential group differences in the use of emotional displays to guide components of social motivation earlier in the course of illness.
Present Study
We tested several hypotheses. First, compared to controls, people in the SSD group would show impaired reward learning, as evidenced by placing less trust in social partners, and anticipate less pleasure over the course of repeated social interactions with positive outcomes. For both reward learning and anticipated pleasure, we predicted that there would be no group differences in the use of negative outcomes to inform these components. Second, compared to controls, people in the SSD would expend less effort to increase the likelihood of future social interactions over the course of interactions with positive outcomes. Given the lack of prior investigation, we explored group differences in effort expenditure for negative social outcomes. Third, we predicted that both groups would use the information signaled by emotional displays to guide components of social motivation. Fourth, for each component, we predicted that impairments in using positive, but not negative social interaction outcomes would be associated with poorer social functioning. In addition to these hypotheses, we explored the inter-relationships between social motivation components in both groups.
Material and Methods
Participants
Sixty-four people met DSM-IV-TR (American Psychiatric Association, 2000) criteria for SSD: schizophrenia (n = 34), schizoaffective (n = 27), or schizophreniform disorder (n = 3). Diagnoses were confirmed using the Structured Clinical Interview for DSM-IV-TR disorders (SCID-IV-TR; First et al., 2002). Participants were between the ages of 18 and 35 and within the first five years of formal diagnosis. Participants had no history of neurological disorders or serious head trauma, were fluent in English, had an estimated IQ > 70, and did not meet criteria for a substance dependence disorder within the past six months. Fifty-five of the 64 participants in the SSD group were taking an antipsychotic at the time of this study. See Table 1 for demographic information. The majority of the SSD group were recruited locally from an early psychosis clinic, and the remainder (n = 26) were remotely recruited from 12 states across the country using online advertisements (Craigslist, message boards, lab website). For remote participants, informed consent was obtained using Qualtrics Insight Platform (Provo, UT) and clinical assessments were conducted via FaceTime or Skype. Informed consent was obtained for all participants prior to completing any study procedures.
Table 1.
Demographic and Clinical variables
| SSD Group (n = 64) | Controls (n = 26) | SSD vs. Control (p) | |
|---|---|---|---|
| Age | 23.02 (3.9) | 25.15 (7.1) | 0.12 |
| Education | 13.78 (2.1) | 13.50 (.91) | 0.51 |
| Sex (M/F) | 47/17 | 16/10 | 0.26 |
| Racial Background (%) | 0.01 | ||
| Caucasian | 48.4% | 46.2% | |
| Asian | 18.8% | 42.3% | |
| Black | 18.8% | 11.5% | |
| Other | 14.0% | 0% | |
| WTAR FSIQ | 107.18 (8.0) | 108.19 (14.6) | 0.68 |
| RFS Social Networks | 4.52 (1.6) | 6.15 (1.6) | < 0.001 |
| Duration of illness (months) | 30.17 (19.0) | -- | -- |
| Chlorpromazine Equivalents | 344.05 (351.9) | -- | -- |
| PANSS Positive subscale | 7.02 (3.7) | -- | -- |
| PANSS Negative Subscale | 13.88 (6.5) | -- | -- |
Note. WTAR = Wechsler Test of Adult Reading Scale; FSIQ = Full Scale IQ; RFS = Role Functioning Scale; PANSS = Positive and Negative Syndrome Scale.
Twenty-six control participants were recruited from an undergraduate research pool. Participants were undergraduate students between the ages of 18 and 35 who received partial course credit for psychology classes and completed the study in-person. Control participants were demographically similar to the SSD group (see Table 1) and did not meet criteria for a current or past mood, anxiety, or psychotic disorder. To better match the demographics of the SSD group, we broadened our recruitment to include non-traditional students whose age fell outside the typical range for college students (18–22). As such, the resulting age range had a bimodal rather than normal distribution, which contributed to the greater variance in age values. All study procedures were approved by an Institutional Review Board.
Self-Report and Interview-based Assessment
Full-scale IQ was estimated using the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001) for all participants. For the SSD group, real-world social functioning was assessed with the Role Functioning Scale (RFS; McPheters, 1984), and positive and negative symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1988), with higher scores indicating greater levels of symptom severity (Wallwork et al., 2012).
Modified Trust Task
After providing informed consent, participants completed a modified version of the Trust Game used in previous studies, which was adapted to an online format using Qualtrics Insight Platform (Provo, UT) for all participants. During this task, participants interacted with four simulated social partners identified by name and a dynamic video of them expressing either an emotional (smile or scowl) or neutral facial display. Participants indicated their anticipated pleasure from the outcome of the interaction (1 to 7 scale) and how many points to send to a partner (between 0 and 10) using the keyboard. The amount of points sent by the participant was then quadrupled and social partners returned a percentage of the quadrupled amount (0% to 100%), with both the participant and social partner’s percentage shown on the screen. Thus, the amount of points sent by a participant represents how much he/she trusted that a social partner would honor and reciprocate his/her trust by returning points (see Figure 1).
Figure 1.
Example of a modified trust task trial. Participants first saw the name and a dynamic video clip of a social partner displaying an emotional (smile/scowl) or neutral facial expression. Next, participants rated their anticipated pleasure for the interaction outcome and then decided how much trust to place in the social partner. Participants then saw the outcome of the interaction and were presented with the option to expend effort to increase or decrease the likelihood of future interactions.
Note. Pairing of social partner display and gender was different across the two study versions. Participants interacted with each social partner 8 times for a total of 32 trials.
Finally, participants could influence the likelihood of interacting with this social partner again in the future by expending effort in the form of repeated key presses. Participants could repeatedly press a specific key to increase the likelihood, a different key to decrease the likelihood, or simply choose to do nothing for the duration of the 6-second response window if they did not have a preference. Participants’ responses did not actually impact the likelihood of future interactions. We averaged the number of key presses across the response window to create an index of the number of key presses per second per participant.
Social partner behavior was predetermined so that interactions with two partners resulted in positive outcomes (average return double the amount sent) while interactions with the other two social partners resulted in negative outcomes (average return half the amount sent). Each social partner’s behavior was consistent throughout the study, but varied from trial to trial (i.e., returned 80% on one trial and 50% on the next trial). The order of interactions was pseudo-randomized so that participants never interacted with the same partner on consecutive trials. The total amount of points a participant received did not accumulate across trials and was reset after each interaction. Participants interacted with each social partner 8 times for a total of 32 trials (see Figure 1).
Social Partner Emotional Displays
The dynamic 5s video clips were chosen from the Amsterdam Dynamic Facial Expression Set (ADFES; Van der Schalk et al., 2011). We chose 4 actors (2 men, 2 women), with one member of each gender expressing an emotion and the other expressing a neutral display. Participants were randomized to one of two study versions, counterbalanced with each version having a different pairing of social partner gender and emotional display. Actor videos (male, female, emotional, neutral) were matched based on ratings from an independent sample on attractiveness, trustworthiness, and emotional intensity. Each social partner exhibited the same display for all interactions.
Statistical Analysis Plan
We first examined the normality of our dependent variable distributions using the Shapiro-Wilk test. Next, we examined between and within group differences in demographic information using t-tests for continuous variables and chi-square tests for categorical variables. To examine changes in decisions to trust, anticipated pleasure, and effort expenditure over the course of repeated social interactions, we used linear mixed effects regression models. The frequency of trials where effort was expended to decrease the likelihood of interactions with positive outcomes and increase the likelihood of interactions with negative outcomes was very low, with more than half of the participants in both groups expending no effort on these trials. Given the low frequency of effort on these trials, we only conducted models for effort to increase the likelihood of interactions with positive outcomes and decrease the likelihood of interactions with negative outcomes.
Linear mixed effects regression models can accommodate the nesting that occurs in the repeated measurement of the same individuals over time by modelling the random distribution of individual differences in level (random effect for intercept) and change over time (random effect for slope). We conducted separate models for interactions with positive and negative social interaction outcomes. Models included the following variables: group (SSD, control), emotion (smile, neutral), time (repeated social interactions), and all possible higher order interactions. Model analyses were conducted using the lme4 package in R version 3.1.0 (R Core Team, 2013). We reported unstandardized beta coefficient estimates, standard errors, and effect sizes (Cohen’s d) for each model effect.
To examine the relationship between impairments in social motivation for interactions with positive outcomes and social functioning in the SSD group, we computed correlations between each component and the RFS Social Network subscale. We investigated the inter-relationships between social motivation components by computing correlations between average decisions to trust, anticipated pleasure, and effort expended to increase or decrease the likelihood of future interactions for each group.
Results
Demographics were not associated with any study variables. In addition, there were no gender differences in task performance nor any interactions between participant and social partner gender for any of the dependent variables within either group. There were significantly more Asian participants in the control compared to SSD group, but racial background was not associated with any study variables. Within the SSD group, there were no demographic or performance differences between participants who were enrolled in-person versus remotely. In addition, there were no performance differences between SSD participants diagnosed with schizophrenia versus schizoaffective disorder. Further, there were no differences between people that were and were not taking anti-psychotic medications and chlorpromazine equivalents were not associated with task performance. Greater negative symptoms were associated with decreased trust (r = −0.33, p < 0.05) and greater effort to decrease the likelihood of future interactions with negative outcomes with both scowling (r = 0.28, p = 0.03) and neutral social partners (r = 0.28, p = 0.03). Positive symptoms were not associated with any components (see Supplemental Materials).
Prior to starting the task, participants rated how positive they found each social partner emotional display to be on a 1 (not at all) to 7 (very much so scale). For both groups, smiling male and female social partners were rated as more positive than their neutral counterparts and scowling male and female social partners were rated as less positive than their neutral counterparts. Next, we compared whether the ratings of how positive smiling, scowling, and neutral social partner emotional displays were different for people with and without a SSD. We found no group differences in the ratings made for smiling, scowling, or neutral facial displays (see Supplemental Materials).
Using Positive Interaction Outcomes to Inform Social Motivation
Anticipated Pleasure
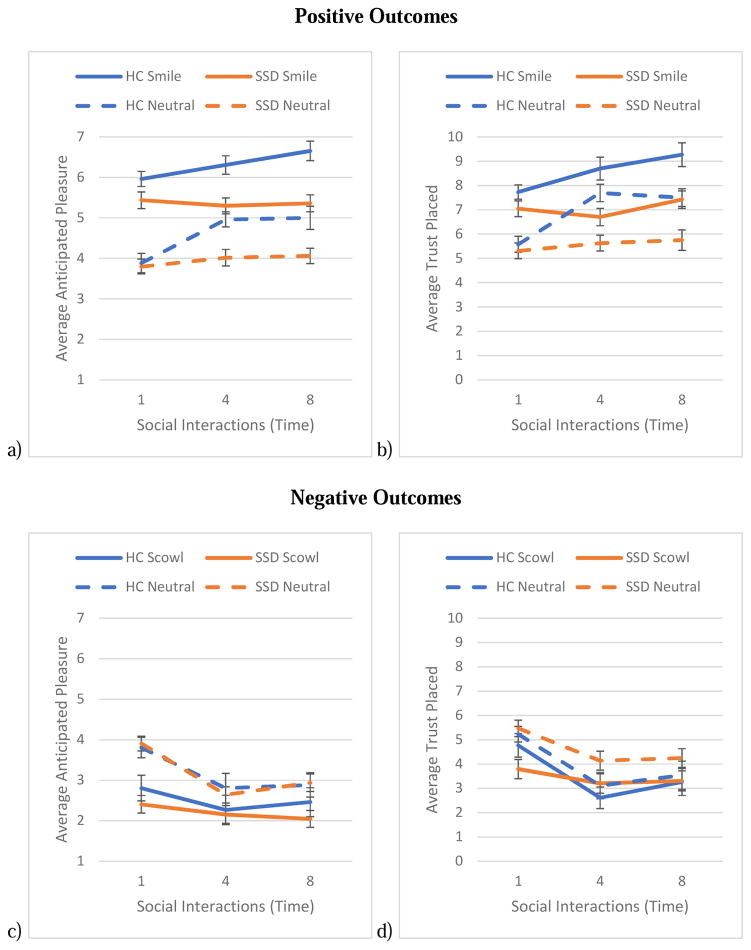
See Table 2 for a complete list of model effect estimates. We found partial support for our hypotheses as the Group × Time interaction approached significance, with the SSD group anticipating less pleasure over the course of repeated social interactions (see Figure 2a). We did find a main effect of emotion, with both groups anticipating greater pleasure from interactions with smiling compared to neutral social partners, t(89) = 8.72, p < 0.001. Greater anticipated pleasure was associated with a trend towards significantly greater real-world social functioning, p = 0.09.
Table 2.
Linear mixed effects regression results for predicted anticipated pleasure and decisions to trust during social interactions with positive and negative outcomes.
| Anticipated Pleasure | Decisions to Trust | Effort Expenditure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Effect | Outcome | B | SE | d [95% CI] | B | SE | d [95% CI] | B | SE | d [95% CI] |
| Intercept | Positive | 4.18 | 0.20 | -- | 6.11 | 0.37 | -- | 3.08 | 0.43 | -- |
| Negative | 3.49 | 0.26 | -- | 4.57 | 0.46 | -- | 2.52 | 0.49 | -- | |
| Time | Positive | 0.09*** | 0.03 | 0.79 [0.31 to 1.25] | 0.16*** | 0.04 | 0.89 [0.41 to 1.36] | 0.10*** | 0.03 | 0.74 [0.27 to 1.21] |
| Negative | −0.06** | 0.02 | −0.56 [−0.14 to −1.03] | −0.09* | 0.04 | −0.52 [−0.08 to −0.97] | 0.10** | 0.04 | 0.61 [0.14 to 1.07] | |
| Group | Positive | −0.17 | 0.24 | −0.16 [−0.62 to 0.29] | −0.51 | 0.44 | −0.27 [−0.73 to 0.19] | −1.05* | 0.51 | −0.48 [−.93 to −0.01] |
| Negative | 0.05 | 0.31 | 0.04 [−0.42 to 0.50] | 0.54 | 0.56 | 0.23 [−0.23 to 0.68] | 0.24 | 0.58 | 0.09 [−0.36 to 0.55] | |
| Emotion | Positive | 1.75*** | 0.25 | 1.64 [1.13 to 2.16] | 1.64*** | 0.38 | 1.01 [0.53 to 1.48] | −0.36 | 0.34 | −.24 [−0.70 to 0.21] |
| Negative | −0.90*** | 0.25 | −0.85 [−0.39 to −1.31] | −0.60 | 0.44 | −0.32 [−0.76 to 0.16] | 0.79* | 0.38 | 0.48 [0.02 to 0.94] | |
| Group × Time | Positive | −0.06^ | 0.03 | −0.44 [−0.90 to −0.02] | −0.12* | 0.05 | −0.53 [−0.99 to −0.07] | −0.08* | 0.04 | −0.48 [−0.93 to −0.01] |
| Negative | 0.02 | 0.03 | 0.16 [−0.29 to 0.62] | 0.03 | 0.05 | 0.13 [−0.33 to 0.59] | −0.12** | 0.05 | −0.60 [−1.06 to −0.13] | |
| Group × Emotion | Positive | −0.11 | 0.29 | −0.09 [−0.55 to 0.37] | −0.11 | 0.44 | −0.06 [−0.51 to 0.40] | 0.06 | 0.04 | 0.35 [−0.11 to 0.80] |
| Negative | −0.23 | 0.30 | −0.18 [−0.62 to 0.28] | −0.70 | 0.53 | −0.31 [−0.74 to 0.18] | −0.51 | 0.45 | −0.26 [−072 to −0.19] | |
| Emotion × Time | Positive | −0.03 | 0.03 | −0.27 [−0.73 to 0.19] | −0.03 | 0.04 | −0.16 [−0.62 to 0.29] | 0.11** | 0.04 | 0.68 [0.21 to 1.14] |
| Negative | 0.03 | 0.03 | 0.27 [−0.19 to 0.72] | 0.01 | 0.05 | 0.01 [−0.29 to 0.29] | −0.11** | 0.04 | −0.59 [−1.05 to −0.13] | |
| Group × Time × Emotion | Positive | 0.001 | 0.03 | 0.01 [−0.47 to 0.44] | 0.02 | 0.05 | 0.08 [−0.54 to 0.37] | −0.09^ | 0.05 | −0.44 [−0.02 to 0.90] |
| Negative | −0.01 | 0.03 | −0.04 [−0.45 to 0.29] | 0.03 | 0.06 | 0.10 [−0.35 to 0.56] | 0.09^ | 0.05 | 0.41 [−0.05 to 0.87] | |
p < .10,
p < 0.05,
p < 0.01,
p < 0.001
Figure 2.
Figure 2 shows the average anticipated pleasure and trust placed over the course of repeated interactions with social partners displaying emotional and non-emotional displays. Figure 2a and 2c show the average anticipated pleasure over the course of repeated interactions with positive (2a) and negative (2c) outcomes. Figures 2b and 2d show the average trust placed over the course of repeated interactions with positive (2b) and negative (2d) outcomes.
Decisions to Trust
In line with our hypothesis, we found a significant Group × Time interaction, with people in the SSD group placing comparatively less trust in social partners over the course of repeated interactions (see Figure 2b). Also in line with our hypothesis, we found a main effect of emotion, with both groups placing more trust in smiling social partners, t(89) = 6.87, p < 0.001. In people with SSD, greater trust during interactions with positive outcomes was associated with significantly greater real-world social functioning, r(64) = 0.25, p = 0.05.
Effort Expenditure
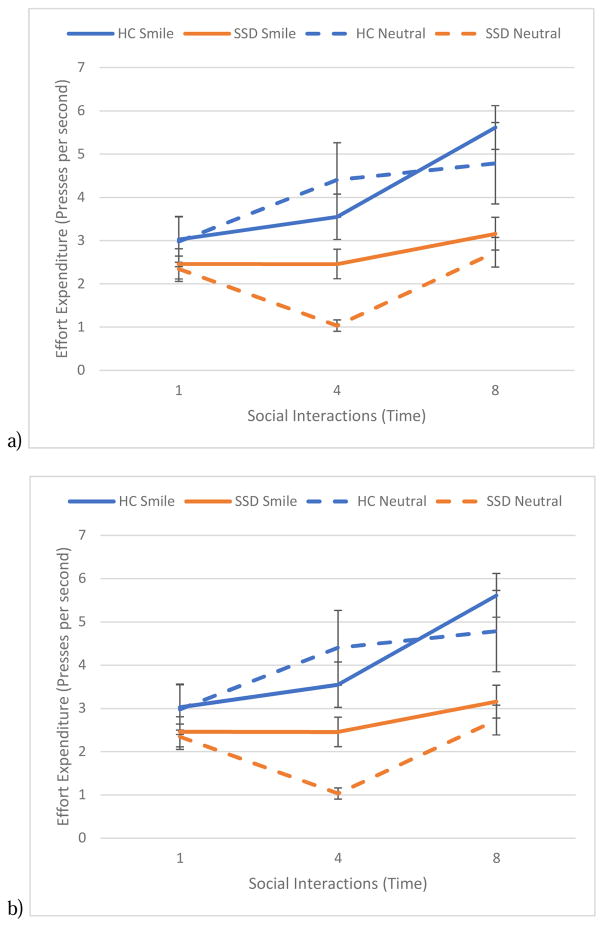
We found support for our hypothesis as the Group × Time Type interaction was significant with the SSD group expending less effort over the course of repeated interactions (see Figure 3a). While we did not find a main effect of emotion, we did find a significant Time × Emotion interactions as both groups expended greater effort over the course of interactions with smiling compared to a neutral social partners. All other effects were not significant. Effort expenditure to increase the likelihood of future interactions was not associated with real-world social functioning (p’s > 0.52).
Figure 3.
Figure 3 shows the average effort expenditure over the course of repeated interactions with social partners displaying emotional and non-emotional displays. Figure 3a shows that, compared to controls, people in the SSD group expended less effort to increase the likelihood of future social interactions with positive outcomes over the course of repeated interactions. Figure 3b, shows that, compared to controls, people in the SSD group expended less effort to decrease the likelihood of future social interactions with negative outcomes over the course of repeated interactions.
Using Negative Interaction Outcomes to Inform Social Motivation
Anticipated Pleasure
In line with our hypotheses, there were no group differences in anticipated pleasure over the course of interactions and both groups anticipated less pleasure from interactions with scowling compared to neutral social partners, t(89) = −6.50, p < 0.001 (see Figure 2c). Anticipated pleasure was not associated with real-world social functioning (p’s > 0.11).
Decisions to Trust
We found partial support for our hypotheses as there were no group differences in decisions to trust social partners over the course of interactions as well as between scowling and neutral social partners (see Figure 2d). The amount of trust was not associated with real-world social functioning (p’s > 0.12).
Effort Expenditure
We found a significant Group × Time interaction with the SSD group expending less effort over the course of repeated interactions (see Figure 3b). We also found a significant main effect of emotion that was qualified by a significant Time × Emotion. However, unlike interactions with positive outcomes, both groups expended greater effort over the course of interactions with neutral compared to scowling social partners. All other effects were not significant. Effort expenditure to decrease the likelihood of future interactions was not associated with real-world social functioning (p’s > 0.22).
Inter-relationships Between Social Motivation Components
In the SSD group, the amount of trust placed in social partners during interactions with both positive and negative outcomes was positively associated with anticipated pleasure, r(64) = 0.64, p < 0.001, but neither the amount of trust placed nor anticipated pleasure during interactions with positive or negative outcomes were associated with effort expenditure (p’s > 0.20). For controls, the amount of trust placed in social partners during interactions with both positive and negative outcomes was positively associated with anticipated pleasure, r(26) = 0.67, p < 0.01. However, unlike the SSD group, both the amount of trust placed, r(26) = −0.39, p = 0.04, and anticipated pleasure, r(26) = −0.56, p < 0.01, during interactions with negative outcomes was negatively associated with effort expended to decrease the likelihood of future interactions with negative outcomes. All other association were not significant (p’s > 0.15).
Discussion
In this study, we examined how people with and without SSD used positive and negative social interaction outcomes and social partner emotional displays to guide multiple components of social motivation as well as how these components work together. In line with our hypotheses, we found that compared to controls, people with a SSD had comparative deficits in reward learning as evidenced by decreased decisions to trust and a trend towards diminished anticipated pleasure for interactions with positive, but not negative outcomes. Trust is a key component in forming and maintaining social relationships and the amount of anticipated pleasure is associated with the likelihood of engaging in a particular course of action (Mellers and McGraw, 2001). Also in line with our hypotheses, we found that impairments in these components were associated with poorer social functioning, which suggests that difficulties learning from positive interaction outcomes and using this information to guide anticipated pleasure and decisions to trust may contribute to the deficits in social functioning found across the schizophrenia spectrum (Fulford et al., 2013; Schlosser et al., 2014). Of importance, we also found a strong, positive relationship between anticipated pleasure and decisions to trust in both groups, regardless of interaction outcome. Thus, for people in the SSD group, it appears that difficulty using positive outcomes to independently inform anticipated pleasure and decisions to trust may also impact how these components work together.
Another factor that may contribute to difficulties establishing and maintaining social relationships among people with SSD is effort expenditure. Our findings suggest that rather than simply choosing to not to expend effort, or expending effort “inefficiently”, as is the case for monetary rewards (McCarthy et al., 2016), people with SSD may be more less willing to expend effort to seek out future interactions with positive outcomes. Interestingly, people with a SSD also expended less effort to decrease the likelihood of future interactions with negative outcomes. This study is the first to examine effort expenditure for negative outcome, and our finding suggests that deficits in effort expenditure may not be outcome specific. One possible explanation for a decrease in effort expenditure among people with a SSD is that effort expenditure appears to be disconnected from other components of social motivation. Therefore, decisions to expend effort among people in the SSD group may be made independent of information from other social motivation components. The potential impact of these findings clearly warrants future investigation to better understand how deficits in effort expenditure influence social behavior among people with SSD. Interestingly, neither group showed a connection between anticipated pleasure or trust and effort expenditure for interactions with negative outcomes. This suggests that the connection between motivation components may be particularly important for informing effort expended for seeking out rewarding social interactions.
Still, there are other possible explanations that should be ruled out to more clearly elucidate the nature of social motivation impairment among those with SSD. For example, people with schizophrenia also have deficits in the updating of internal representations of reward in order to inform behavior (Gold et al., 2008; Kring and Barch, 2014), as well as maintaining emotional experiences (Kring et al., 2011; Ursu et al., 2011). Deficits in either value representation or the maintenance may contribute to the dissociation between emotional experience and motivated behavior found in previous studies of non-social motivation (Heerey and Gold, 2007; Lui et al., 2016). Thus, while our study extended previous investigation to the social world, future studies should continue the simultaneous investigation of multiple social motivation components to better understand their contributions to impairment.
Impairments in social motivation, however, do not appear to be due to difficulties using the information signaled by social partner’s emotional displays. Indeed, in line with our hypothesis, both people with and without SSD used the information signaled by smiles to increase anticipated pleasure, trust, and effort expenditure. However, while both people with and without schizophrenia anticipated less pleasure during interactions with scowling compared to neutral social partners, there were no differences in the amount of trust placed during interactions with these partners. Furthermore, while both groups expended greater effort during interactions with scowling compared to neutral partners, there was a greater decrease in this effort expenditure over the course of repeated interactions. Thus, it appears that the information signaled by scowls did not influence the decisions to trust and the continued expenditure of effort expenditure during these interactions. Future studies should continue seek to unpack how the information signaled by emotional displays is used to guide components of social motivation and how this information changes over the course of repeated interactions.
Taken together, our findings have implications for our understanding of social motivation in the schizophrenia-spectrum. Models of motivation in schizophrenia (e.g., Barch and Dowd, 2010; Kring and Barch, 2014) posit that individual components work together to produce motivated behavior, and that impairments in specific processes contribute to motivational impairment. However, a recent review in people with schizophrenia proposed that underlying impairments in components of motivated behavior could be due to aberrant interactions between cortical-striatal brain regions, implicating neural network disconnection as a possible cause (Strauss et al., 2014). Our findings provide behavioral evidence for both accounts; people with a recent-onset SSD not only experienced impairments in individual components of social motivation for interactions with positive outcomes, but these components appear to be connected differently than in people without a SSD. An important next step will be to take this work outside of laboratory and into the daily lives of people with SSD. Indeed, real-world social interactions are often more variable and unpredictable, with outcomes changing from interaction to interaction and vacillating between rewarding (e.g., acceptance, reciprocation) and punishing (e.g., rejection) outcomes and examining how these motivational components work together in everyday life has critical implications for understanding social deficits in schizophrenia.
Our study had some limitations. First, we asked participants to consciously rate their anticipated pleasure and make decisions about trust and effort expenditure, which are processes that typically occur at a much faster pace and outside of conscious awareness. As such, future studies should test whether impairments persist in situations that require implicit rather than explicit use of social motivation components. Second, while we examined correlates of social motivation impairment, there may be other individual differences, such as altruism, that influenced decisions to trust and other motivation components. As such, future studies should consider further examining factors that be related to or moderate social motivation. Third, while study trials involved an interaction with a social partner, the outcomes were expressed as points. To increase the social nature of interaction outcomes, future studies might consider using other kinds of social outcomes, such as smiles/scowls and acceptance/rejection. In addition, while we used the word interaction to describe participant/social partner exchanges and incorporated dynamic facial stimuli to represent social partners, trials in this task lacked the back and forth found in traditional social interactions. Future studies should consider ways to increase the ecological validity of investigating components of social motivation.
We designed this task so that participants had the option to wait for the 6 second period to end, and thus effort expenditure was volitional. However, it may have been the case that effort expended to decrease future interactions with positive outcomes and increase interactions with negative outcomes, which was very low overall, was due to a lack of paying attention or confusion on behalf of people in the SSD group. Fourth, while the control group was demographically similar to the SSD group, the higher level of functioning that is typical of college undergraduates may make the findings from this group less generalizable. Finally, while we did not assess working memory in this current study, previous studies have not found any link between working memory and task performance in people with schizophrenia (Campellone et al., 2016).
In summary, we found that compared to controls, people with a recent-onset SSD have impairments in multiple components of social motivation. Specifically, people with a recent-onset SSD placed less trust, tended to anticipate less pleasure, and expended less effort to increase the likelihood future interactions with positive social outcomes. Importantly, impairments in components of social motivation appear to be specific to interactions with positive outcomes, with the only group difference for interactions negative outcomes being decreased effort expenditure by people with a SSD. Furthermore, impairments in social motivation are not due to difficulties using the information signaled by social partner emotional displays. Taken together, our findings provide potential intervention targets for enhancing social motivation during a critical period of social development.
Supplementary Material
Highlights.
People with schizophrenia often experience impairments in components of motivation
This includes anticipated pleasure, reward learning, and effort expenditure
We extended previous work to motivation for social interactions
We show that impairments are largely specific to motivation for positive interactions
Impairments may also result from disconnection between motivation components
Acknowledgments
We are grateful to the participants to thank all the participants in this study.
Financial Support
US National Institutes of Mental Health grant 5T32MH089919 to TRC and US National Institutes of Mental Health grants R34MH110583 and K23MH97795 to DAS.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: The role of prefrontal-striatal interactions. Schizophr Bull. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 2014;123:387–397. doi: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone TR, Fisher A, Kring AM. Using social outcomes to inform decision-making in schizophrenia: Implications for symptoms and functioning. J Abnorm Psychol. 2016;25:310–321. doi: 10.1037/abn0000139. [DOI] [PubMed] [Google Scholar]
- Campellone TR, Kring AM. Anticipated pleasure for positive and negative social interaction outcomes. Psych Res. doi: 10.1016/j.psychres.2017.09.084. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WC, Waltz JA, Gold JM, Chan TCW, Chen EYH. Mild reinforcement learning deficits in patients with first-episode psychosis. Schiz Bull. 2016:sbw060. doi: 10.1093/schbul/sbw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- de la Asuncion J, Docx L, Sabbe B, Morrens M, de Bruijn ER. Converging evidence of social avoidant behavior in schizophrenia from two approach-avoidance tasks. J Psych Res. 2015;69:135–141. doi: 10.1016/j.jpsychires.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Engel M, Fritzsche A, Lincoln TM. Anticipation and experience of emotions in patients with schizophrenia and negative symptoms. An experimental study in a social context. Schizophr Res. 2016;170:191–197. doi: 10.1016/j.schres.2015.11.028. [DOI] [PubMed] [Google Scholar]
- Fett AJ, Shergill SS, Korver-Nieberg N, Yakub F, Gromann PM, Krabbendam L. Learning to trust: trust and attachment in early psychosis. Psychol Med. 2016;46:1437–1447. doi: 10.1017/S0033291716000015. [DOI] [PubMed] [Google Scholar]
- Fett AJ, Shergill SS, Joyce DW, Riedl A, Strobel M, Gromann PM, Krabbendam L. To trust or not to trust: the dynamics of social interaction in psychosis. Brain. 2012;135:976–984. doi: 10.1093/brain/awr359. [DOI] [PubMed] [Google Scholar]
- Fett AK, Shergill SS, Krabbendam L. Social neuroscience in psychiatry: unravelling the neural mechanisms of social dysfunction. Psychol Med. 2015;45:1145–1165. doi: 10.1017/S0033291714002487. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, William JBW. Structured Clinical Interview for DSM-IV-TR Axis 1 disorders, Research Version, Patient Edition (SCID-I/P) New York, NY: New York State Psychiatric Institute, Bethesda, MD; 1992. [Google Scholar]
- Frost KH, Strauss GP. A review of anticipatory pleasure in schizophrenia. Curr Behav Neurosci Rep. 2016;3:232–247. doi: 10.1007/s40473-016-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford D, Niendam TA, Floyd EG, Carter CS, Mathalon DH, Vinogradov S, Stuart BK, Loewy RL. Symptom dimensions and functional impairment in early psychosis: more to the story than just negative symptoms. Schizophr Res. 2013;147:125–131. doi: 10.1016/j.schres.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, Vinogradov S. Do people with schizophrenia have difficulty anticipating pleasure, engaging in effortful behavior, or both? J Abnorm Psychol. 2014;123:771–782. doi: 10.1037/abn0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psych. 2013;74:130–136. doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, Gollins A, Frank MJ. Negative symptoms and the failure to represent the expected reward value of actions: Behavioral and computational modeling evidence. Arch Gen Psych. 2012;69:129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Horan WP, Barch DM, Gold JM. Effort-based decision making: A novel approach for assessing motivation in schizophrenia. Schizophr Bull. 2015;41:1035–1044. doi: 10.1093/schbul/sbv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerdink MW, van Kleef GA, Homan AC, Fischer AH. Emotional expressions as social signals of rejection and acceptance: Evidence from the affect misattribution paradigm. J Exp Soc Psychol. 2015;56:60–68. [Google Scholar]
- Heerey EA. Learning from social rewards predicts individual differences in self-reported social ability. J Exp Psychol Gen. 2014;143(1):332. doi: 10.1037/a0031511. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol. 2007;116:268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacology. 2014;24(5):725–736. doi: 10.1016/j.euroneuro.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Elis O. Emotion deficits in people with schizophrenia. Ann Rev Clin Psychol. 2013;9:409–433. doi: 10.1146/annurev-clinpsy-050212-185538. [DOI] [PubMed] [Google Scholar]
- Lui SSY, Liu ACY, Chui WWH, Li Z, Geng F, Wang Y, Heerey EA, Cheung EFC, Chan RCK. The nature of anhedonia and avolition in patients with first-episode schizophrenia. Psychol Med. 2016;46:437–447. doi: 10.1017/S0033291715001968. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Ambady N, Kleck RE. The effects of fear and anger facial expressions on approach-and avoidance-related behaviors. Emotion. 2005;5(1):119. doi: 10.1037/1528-3542.5.1.119. [DOI] [PubMed] [Google Scholar]
- McCarthy JM, Treadway MT, Bennett ME, Blanchard JJ. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr Res. 2016;170:278–284. doi: 10.1016/j.schres.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters HL. Statewide mental health outcome evaluation: A perspective of two southern states. Comm Ment Health J. 1984;20:44–55. doi: 10.1007/BF00754103. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Grunder G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49:3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Robertson BR, Prestia D, Twamley EW, Patterson TL, Bowie CR, Harvey PD. Social competence versus negative symptoms as predictors of real world social functioning in schizophrenia. Schiz Res. 2014;160:136–141. doi: 10.1016/j.schres.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharlemann JPW, Eckel CC, Kacelnik A, Wilson RK. The value of a smile: Game theory with a human face. J Econ Psychol. 2001;22:617–640. [Google Scholar]
- Schlosser DA, Campellone TR, Biagianti B, Delucchi KL, Gard DE, Fulford D, Stuart BK, Fisher M, Loewy RL, Vinogradov S. Modeling the role of negative symptoms in determining social functioning in individuals at clinical high risk of psychosis. Schizophr Res. 2015;169:204–208. doi: 10.1016/j.schres.2015.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser DA, Fisher M, Gard D, Fulford D, Loewy RL, Vinogradov S. Motivational deficits in individuals at-risk for psychosis and across the course of schizophrenia. Schizophr Res. 2014;158:52–57. doi: 10.1016/j.schres.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psych. 2011;69:424–431. doi: 10.1016/j.biopsych.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40:S107–S116. doi: 10.1093/schbul/sbt197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Peterman JS, Zald DH, Park S. Impaired effort allocation in patients with schizophrenia. Schizophr Res. 2015;161:382–385. doi: 10.1016/j.schres.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Gard MG, Minzenberg MJ, Yoon JH, Ragland JD, Solomon M, Carter CS. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. Am J Psych. 2011;168:276–285. doi: 10.1176/appi.ajp.2010.09081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Schalk J, Hawk ST, Fischer AH, Doosje BJ. Moving faces, looking places: The Amsterdam Dynamic Facial Expressions Set (ADFES) Emotion. 2011;11:907–920. doi: 10.1037/a0023853. [DOI] [PubMed] [Google Scholar]
- Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137:246–250. doi: 10.1016/j.schres.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. Psychological Corporation; London: 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.